|
|
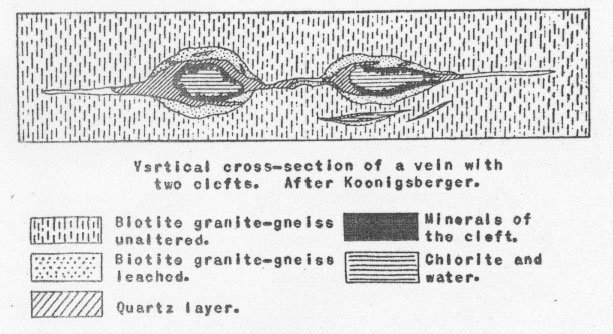
Volume 12, pages 157-167, 1927 ALPINE MINERAL DEPOSITS IAN CAMPBELL, Louisiana State University. For many years past a considerable literature has been developing about the Swiss "Alpine Mineral Deposits." Recently a paper1 by Parker has appeared giving an excellent general summary of the present state of knowledge of these interesting deposits, and it has seemed worth while to make a rather extended review of this work. From the historical standpoint alone the deposits are interesting since they were among the first to be studied and have been among the most important in the development of the science of mineralogy. From these deposits came the "super-frozen" ice, which the ancients called "Krystallos," giving our science the name crystallography. But it is more than a name that crystallography owes to this "ice stone," for it has been from similar specimens of this quartz that many of the laws, the complexities and the simplifications of the modern science have been developed. And it is not only the quartz of these deposits that has made them famous; superbly crystallized specimens of many other species, adularia, hematite, epidote, titanite, etc., from these occurrences are known in collections all over the world. If the contribution of these deposits to the science has been great in the past, even greater things are promised for the future. In this further development, crystallography will be by no means the only gainer, for some years ago the Swiss mineralogists led by Koenigsberger recognized the wider geological and mineralogical significance of the deposits; and now, under the leadership of Niggli, a group has been organized to study these deposits from various points of view: crystallographic, paragenetic, chemical and physico-chemical. Altogether it should prove a fruitful field of research. The title "Alpine Mineral Deposits" may properly be applied to the unique occurrences with which this paper is concerned, since no other of the infrequent and unimportant mineral deposits of the Alps is deserving of it. The "Alpine Minerals" are not only the most interesting and the most characteristic but are also actually the most important deposits of the region from an economic standpoint, as many are being actively prospected by collectors today. The deposits of these "Alpine Minerals" are widely distributed throughout the Tyrol, the French and the Swiss Alps. Perhaps the most important general feature of the deposits is their relation to structure. In shape they tend to be lens-like, usually a very much flattened lens. This lens is always in cross-cutting (perpendicular) relationship to the rock structure in which it occurs. The country rock is generally schist or gneiss. Chemical composition of the country rock may vary widely, from the equivalent of granite to that of olivine gabbro, though the salic types are by far the more common. In these rocks the plane of schistosity is very often vertical, or nearly so, and the plane of the deposit then is roughly horizontal, following the transverse tensional cracks produced by the tectonic forces responsible for most of the Alpine structure. (See figure.)
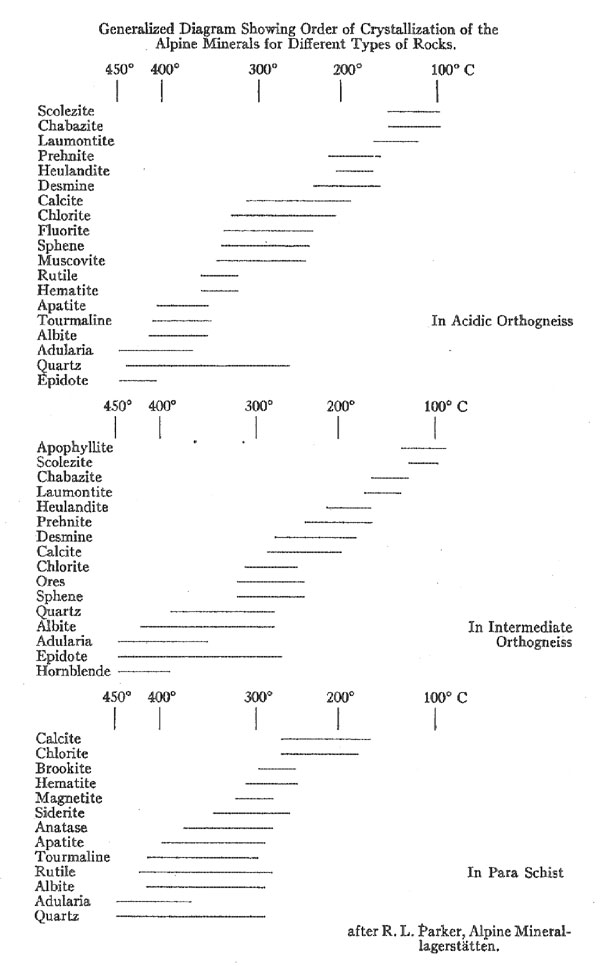
In size they vary from the merest cracks to open crevices 6 to 8 feet in their shortest dimension, and 50 to 100 feet in their two longer horizontal dimensions. Now, while individual occurrences vary widely, there is, nevertheless, a notable similarity between all deposits in the general outline of their structure and mineralogy. They all show a zoning, concentric to the "lens," and in most of the deposits, three such zones can be recognized. The outermost zone, that farthest from the center of the crevice, consists simply of leached country rock, and naturally grades outward into unaltered gneiss or schist as the case may be. Leaching is always most severe in the direction of the open space, and in many cases so much of the rock material has been removed that the remainder has become an exceedingly porous and even friable mass. In width, the leached zone never exceeds double the width of the adjoining open cavity. The most interesting feature about this leached zone is that its width is always proportional to the size of the crevice. The larger the crevice, the greater the width of the leached zone. This noteworthy relationship is true not only in respect to the size (i.e. width) of the two succeeding zones, but also holds good for the size of individual crystals formed in the innermost zone. All of these factors, the width of the three concentric zones, the size of individual crystals and even the relative power of the leaching solutions seem to be directly dependent on the size of the crevice itself. The zone lying immediately within the leached zone (in the direction of the open cavity) is called the quartz zone or quartz layer. By this is meant the large mass of quartz found deposited in part upon the rock or its decomposition product and in part even within it. This mass of quartz is the preponderant part of the "vein" filling, in some cases. making up the entire crystallization within the crevice. Indeed, the only case where it is absent is where the crevice has formed in relatively basic rocks. This quartz zone consists of a mass of rather loosely interlocking, tiny, idiomorphic quartz crystals. While they have not been studied as carefully as the larger crystallizations of the inner zone, it is believed that these, too, are all of the alpha variety of quartz, having formed below 575° C. The third and last zone, the one, therefore, that forms the innermost filling of the crevice, and the one usually referred to when speaking of Alpine "Veins," immediately adjoins the quartz layer. It is the one characterized by the superb crystallizations which have made Alpine minerals the standards for crystallographers. Evidently these minerals must have been deposited from a very slowly cooling solution. The crystals are attached in rather irregular and haphazard fashion to the quartz layer, and project into the free open space of the crevice. They are found growing from both the roof and the floor of the crevice, and gravity seems to have played no part in influencing crystallization or location. An exception is the case of such a mineral as chlorite, the fine flakes of which evidently remained floating in suspension for some time after crystallization, eventually to settle more abundantly on, the crystals growing from the floor of the crevice. A remarkable feature of the attachment of these large crystals is that many are so delicately suspended that the least touch or tremor dislodges them. Now, since there is no indication that. crystals have fallen, either during their period of growth or later, it would seem evident that this entire formation must have postdated all of the tectonic movements of the Alpine region. The assemblage of minerals formed in this zone is unique. (See appended table for complete list). It may perhaps be compared to the suite from some pegmatites and certain hydrothermal veins - and yet the points of difference are many. The order of succession of the minerals has been well established from studies on many of these crevice deposits. In general, it is the same in all, and so far as now known the phenomenon of recurrence of crystallization is entirely lacking. Whenever the deposition of a mineral started, it continued uninterruptedly until the supply was exhausted. This feature, even without the remarkable crystallizations, would set these deposits apart from most hydrothermal veins. Space does not permit a detailed discussion of the various mineral species. Because, however, of its abundance and importance, certain features of the quartz crystals found in this zone will be mentioned. On these crystals the positive and negative rhombohedrons are usually very unequally developed and the faces of the trigonal pyramid and trigonal trapezohedron can nearly always be discerned. These features are usually taken to indicate the low temperature, alpha, variety of quartz. Inclusions of liquid and gas bubbles, and of various minerals, notably chlorite and golden brown rutile needles are very common. Quartz of the smoky variety is common too, and Koenigsberger has made the interesting observation that the degree of coloring or smokiness is dependent upon elevation above sea level. Thus, up to 1400 meters, the quartz remains colorless; at about 1500 meters a brownish color is perceptible; at 1860 it has become distinctly brown; at 2300 it is really smoky quartz; and at 2900 meters it is the deep blue-black variety known as morion. Now x-rays or the gamma rays of radium emanations are known to produce this coloring effect; and the variation in color with altitude is thought to be due in part to the changing effect of the radioactive material in the rocks and in part to the much greater seasonal changes in temperature at the higher altitudes. In looking over the appended list of minerals, it can be seen that the chemical elements present are the same as those that make up the preponderant constituents of the rocks themselves; Al, Fe, Ti, Na, K, Ca, and Al combined with SiO2. Quantitatively as well, there has been found to be a close agreement between the proportions of elements present in the country rock and in the crevice filling. Such a rare element as fluorine, for instance, was found to be present in the unaltered country rock to an amount of 0.06%. In the crevice filling the percentage was 0.05. This remarkable qualitative and quantitative agreement. leads naturally to the assumption that the rocks in which the crevices have been found have themselves furnished the material for the filling. Considering that there has been little if any change in qualitative and quantitative chemical composition it will be interesting to note what mineralogical changes have taken place. Obviously, the material obtained from the rock has undergone a complete rearrangement before the deposition of the new minerals, since they are in the main so different. The outstanding difference is in the almost complete absence in the crevice filling of the feldspars (except the two alkali feldspars, and even these show a striking change in habit) and of all the common femic rock-forming silicates. What, then, becomes of these minerals? One group is made up of minerals which are soluble, but have stable molecules. As might be expected, they appear in chemically similar form in both rock and crevice filling. A small proportion of the quartz probably belongs to this group, as does orthoclase feldspar in part. Muscovite, apatite, fluorite, and titanite may also be included here. It is significant that this group is not the one of major importance as regards the crystallizations of the inner zone. Another group are those rock minerals which are also soluble, but possess less stable molecules. It is this group which has furnished most of the material for the crevice filling. The calcic plagioclases, for instance, undergo a rather complete disintegration with subsequent formation of muscovite, zoisite, zeolites, quartz and, in extreme cases, carbonates. The original plagioclase contains some KAlSi3O8, and when decomposed this goes to form adularia. The formation of zeolites appears to be particularly dependent upon the presence of calcic plagioclase in the rock, as might be expected, since Ca is an essential part of the molecule of nearly all of these zeolites. The presence of K and Na in the depositing solution also seems necessary for the formation of zeolites. It is significant that it is in the crevices formed in dominantly alkaline rocks that the zeolites are most abundant. The biotite of the rocks varies greatly in its composition, and consequently gives rise to a variety of products. The principal crystallization resulting from its decomposition is chlorite. Probably hematite, additional quartz, and some carbonates may be formed. Most of the titanium of the rocks is probably in the biotite and pyroxenes, and to them then, is to be attributed the rutile, anatase, and brookite appearing in the vein. Much of the formation of fluorite too is undoubtedly dependent on the decomposition of the biotite, since its fluorine content is known to be relatively high in these rocks. Hornblende and pyroxene have much the same story as biotite. They may form some of the epidote, and part of the hornblende passes over to actinolite and amianthus (an exceedingly fine silky variety of asbestos). A third group consists of insoluble minerals. Strictly speaking, of course, there is none actually so. But such minerals as quartz and the purer varieties of orthoclase may be grouped here since they remain in large part in the leached rock and form the insoluble residuals, the porous friable mass of the outermost zone. With this survey of the features of the Alpine deposits we are ready to sum up the points in the theory of origin proposed for them. First of all, of course, came the formation of these transverse rifts in the rocks, to be explained perhaps partly by thrust faulting and partly by tensional stresses. Then came the filling of these cavities by waters soaking in from the surrounding rocks or along the extensions of the rifts. The origin of these waters is doubtful. Parker suggests that they may have been largely of magmatic origin, citing the propinquity of metalliferous veins and the presence of porphyry dikes in the region as proof that the magmatic zone once reached near or to the surface and could therefore have been capable of furnishing the water. It is admitted that ground water percolating downward may have been in part responsible, though it is not apparent why to ground water should not have been assigned the entire part. However, it matters not so much from whence the waters came. The important fact is that they brought nothing with them but CO2 and heat, both easily explainable whatever their origin. CO2 is a common constituent of both ground water and igneous emanations With the crevice filled with hot carbonated water, solution of the adjoining rock material began immediately. Leaching continued until a maximum concentration in the water solution had been reached for the prevailing temperature. Any cooling from this stage would cause precipitation, and the beginning of the formation of the "vein," but it would naturally mean an end of leaching. The solution thus formed can be treated just as a complex mixture of salts dissolved in water. Deposition is controlled entirely by falling temperature. The succession of minerals is strictly a salt sequence, comparable in every way to the salt deposits of evaporating sea water and, indeed, they have been studied in much the same way as the Stassfurt deposits. Doelter and Koenigsberger have performed experiments on natural minerals of the rocks which tend to show as well as could be expected, that hot carbonated waters do dissolve these rock minerals, and that the resultant solution will deposit its material in just such fashion as these Alpine minerals have been deposited. Because of the limitation of the time factor, these experiments have never been quite as conclusive or satisfactory as might be desired. More important is the fact that the deposits, or rather the behavior of such a theoretical solution as they are assumed to have come from, has been studied by aid of the phase rule and equilibrium diagrams, with the result that the sequence has been accurately predicted. It is true that in the theoretical sequence, certain compounds occurred not known in the Alpine deposits or even in nature. Yet the fact that these are not known, or rather, perhaps, have not been found, does not reflect on the importance of this study in confirming the "salt sequence" theory of deposition for these deposits. Indeed some of these synthetic "minerals" with no known natural counterparts have been proved to be meta-stable forms and therefore not likely to occur in nature. Last there remains to be considered the temperature range over which deposition occurred. (See appended table). For this only the upper and lower limits can be fixed at all definitely. Other points in the series have been determined largely by interpolation. Quartz and adularia are the oldest formations. Much of this quartz is later than the adularia, it is true, but a part is definitely earlier. Mention has already been made of the fact that all of the Alpine quartz is the alpha, or low temperature variety. It must, then, have formed below 575° C. For adularia, the lowest temperature of formation is known to be 340°C. plus or minus 20°C. The first deposition must therefore have taken place between 500° and 350°C. Now, toward the close of the adularia period, a much larger deposition of other minerals begins to appear, so that evidently the larger part of the deposits was formed from 350°C, downward. Experiments made on the disappearance of the smoky color of quartz and of the "bubble line" in the quartz with inclusions when subjected to heating confirm these temperatures. The lower temperature limits are determined principally by the zeolites. In connection with the synthetic work on these complex hydrous silicates, it seems proved that zeolites can form only from solutions whose chemical composition is very similar to the solid phase which separates. Considering this, it is interesting to note in the natural sequence that zeolites do not begin to appear until most of what might have been interfering compounds have been exhausted. They appear just about the time that calcite, fluorite, and hematite are finishing their crystallization, a further proof of the correctness of the general theory. Synthetically, zeolites have been formed at temperatures as low as 90°C. Their upper limit is just about 300°C. In general the zeolites develop most abundantly at the lower temperatures, their typical range is 100° to 200°C. In these deposits it seems unlikely that the lower limit was ever quite reached, so the lower limit is set at 130°C. Two other points on the temperature range are accurately known. The red color frequently found in the fluorite disappears on heating to 175°C. The formation of fluorite must therefore lie below this. And, finally, hematite is known to be deposited from water solution at about 200°C. The temperature limits, then, between which deposition has taken place, may be set between 500° and 130°C., with most of the deposition occurring in the interval between 350° and 150°C. Finally, to sum up the remarkable features of these deposits, all of which point toward the "lateral secretion" theory, we have: First; the dependence in size both of the leached zones, of the "vein" filling and of individual crystals upon the size of the crevice. Second; the remarkable dependence of the chemical character of the "vein" filling on the chemical character of the country rock. Third; the mineralogical changes, molecular simplification, and hydration, indicative of hydrothermal action. Fourth; the evidence of a "salt sequence" type of deposition controlled by progressively declining temperatures. This article of Parker's is to be regarded as particularly timely, since in these days when the pendulum of popular opinion is swinging toward the direct igneous explanation for all sorts of deposits, it affords a very clear cut and unequivocal example of lateral secretion - lateral secretion in the strictest sense. And, too, because it presents a detailed picture of the conditions of that deposition, a deposition which, while differing in structure and sequence and individual crystallizations, yet is comparable in mineral assemblage to many hydro-thermal veins or "vein dikes," and which indeed, in point of variety of species present, rivals even some of the pegmatites. The pioneer in the foregoing theory of the origin of the Alpine mineral "crevices" is Koenigsberger, who has also collected most carefully the data upon which it rests. Among other papers by him may be mentioned: Die Minerallagerstatten im Biotit protogin des Aarmassivs, Jahrb. für Min., B.B. 14, p. 43; and Die Paragenesis der Kieselsaure-mineralien in Doelter's HANDBUCH DER MINERALCHEMIE, Vol. 11, 1, p. 27. Further papers on this subject from Niggli's group of investigators should be looked forward to by all who are interested in these classic deposits - deposits which bid fair to become a Rosetta stone of Geology. In conclusion the writer wishes to express his thanks and appreciation to Dr. Palache for first calling his attention to this interesting field; for help at many stages in the preparation of this review; and for the privilege of studying the Alpine suite of minerals in the Harvard collection. LIST OF MINERALS APPEARING IN THE "ALPINE VEINS"
NOTES 1 Alpine Minerallagerstätten, Robert L. Parker. Schweizer Mineralogische und Petrographische Mitteilungen, No. 3, 1923, 298. [var:'startyear'='1927'] [Include:'footer.htm'] |