|
|
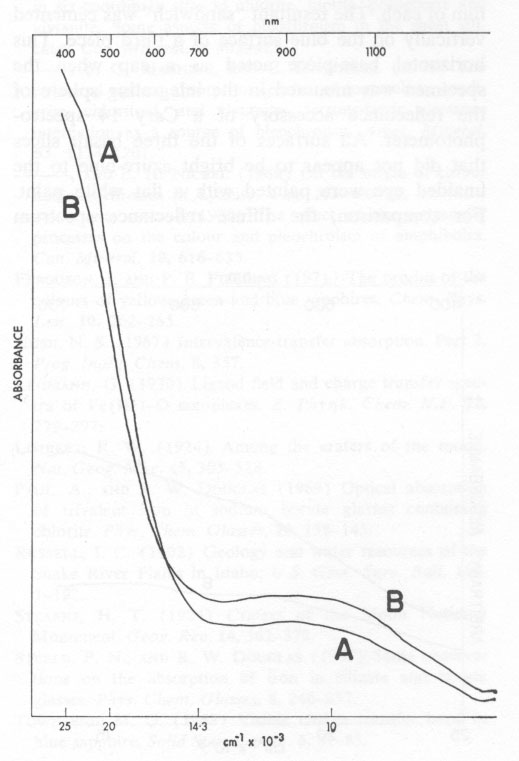
Volume 58, pages 1048-1051, 1973 "Blue Dragon" Basalt from Craters of the Moon National Monument, Idaho: Origin of Color GORDON H. FAYE Mineral Sciences Division, Mines Branch, ROY M. MILLER Sciences Division, Henry Ford Community College, Abstract The unusual color of the "Blue Dragon" lava is due to intense blue light being reflected from clusters of tiny titanian magnetite crystals, which, together with crystallites of plagioclase and olivine, are dispersed throughout an outer layer of clear brown glass. It is proposed that Fe2+→ Fe3+, and probably Fe2+→ Ti4+, electron transfer processes are responsible for the blue color. Introduction Surfaces of a thin pahoehoe lava-flow, extending southwest from the base of Big Cinder Butte in Craters of the Moon National Monument, Idaho, reflect various shades of blue light. This striking phenomenon was first noted by Russell (1902) and the name "Blue Dragon" was established for this particular lava tongue by Limbert (1924) and by Stearns (1924); however, a satisfactory explanation of the color has not been made. The outer layer of the lava consists of microphenocrysts (<300 µm) of plagioclase, olivine, and titanian magnetite dispersed in a clear brown glass matrix; plagioclase predominates in volume among the crystallites. Near the surface, the basalt-glass layer appears inky-blue in reflected light to depths up to 3 mm. This blue layer grades with depth into a brown basalt glass (Fig. 1) and ultimately into opaque basalt. Much of the surface of the Blue Dragon lava is covered by a thin (<5 µm) amorphous film whose apparent color ranges from pale azure-blue to deep inky-blue. Throughout this surface film numerous microphenocrysts of plagioclase, olivine, and magnetite crop out. Casual examination suggests that the film is intrinsically a blue material and that there is no apparent correlation with the inky-blue sub-surface glass layer. However, this conclusion is erroneous as will be explained subsequently. Thin sections of the clear glass matrix from both the blue and the brown sub-surface zones are brown in transmitted light (see Fig. 2). That the outer zone is blue in reflected light and brown in transmitted light is a unique property of the Blue Dragon basalt which has puzzled previous workers. It is here proposed that the striking shades of blue result from reflection of light at the surface of abundant, partly-oxidized magnetite particles in the outer layer of the lava and that the blue color results from either Fe2+→Fe3+ electron transfer alone, or in conjunction with an Fe2+→ Ti4+ electron transfer process. Association of Blue Color with Magnetite In thin sections examined under the microscope in transmitted light, individual grains in the clusters of magnetite are seen to be black and their outline is sharply defined. However, close examination in oblique reflected light of the blue layer of a polished section at approximately 50x (Fig. 1) distinctly reveals inky-blue blotches around the subsurface magnetite clusters, these making the outline of the individual grains rather fuzzy. Such blotches are not associated with the inclusions of plagioclase or olivine; indeed, whitish haloes are evident around the latter. In the "normal" brown basalt, the magnetite inclusions are relatively sharply outlined and do not have extensive blue haloes. Examination of Thin Siliceous Film Similarly, close examination of the outer surface of the lava, under bright light and relatively high power, reveals intense blue blotches around most of the exposed magnetite clusters. The intensity and the shade of blue of the surface is directly related to the intensity of the color of the glass layer just below the surface. This indicates that blue light is being reflected from magnetite particles near the surface and is transmitted and scattered by the thin amorphous film. Under normal conditions of observation, the resultant impression is that the thin siliceous film itself is the source of the intense blue color. However, the blue blotches surrounding the exposed magnetite particles become even more evident after cleaning the specimens with acetone, and then water, in an ultrasonic bath for a few minutes. Qualitative analysis with a laser microprobe (Dr. R. J. Traill of the Geological Survey of Canada) has shown that the surface film contains iron, silicon, and aluminum as major constituents; and magnesium, calcium, and titanium as minor constituents. It is probable, therefore, that the film is an alteration product of the glass and that its composition is similar to the glass. The Sub-Film Glass Matrix By electron microprobe analysis, the iron and titanium contents of the sub-surface glass matrix were found to be 11.0 percent and 1.4 percent respectively, for both the blue and brown zones (Fig. 1). Thus, the difference in the color of the zones cannot be related to differences in the total concentration of these elements. Absorption spectra A and B in Figure 2 are, respectively, those of thin sections of glass taken from the blue and brown layers of the Blue Dragon basalt. These were measured using a microscope-spectrophotometric technique (Faye and Nickel, 1970). The absorption envelope centered at ~1000 nm in the spectra of Figure 2 is certainly due to six-coordinate Fe2+ (e.g., Faye, 1968). Furthermore, from a knowledge of extinction coefficients appropriate for Fe2+ in ferromagnesian silicates, it would seem that most of the iron in the glass is in the ferrous condition. The steeply sloping portion of each spectrum between 400 nm and 600 nm is typical of iron-bearing glasses (Steele and Douglas, 1965; and Paul and Douglas, 1969). For these, the spin-forbidden bands of Fe2+ and Fe3+ (six- and/or four-coordinate) are superimposed on the low-energy wing of the O2- → Fe3+ electron transfer band whose maximum (Lehmann, 1970) is in the ultraviolet between 200 and 250 nm.
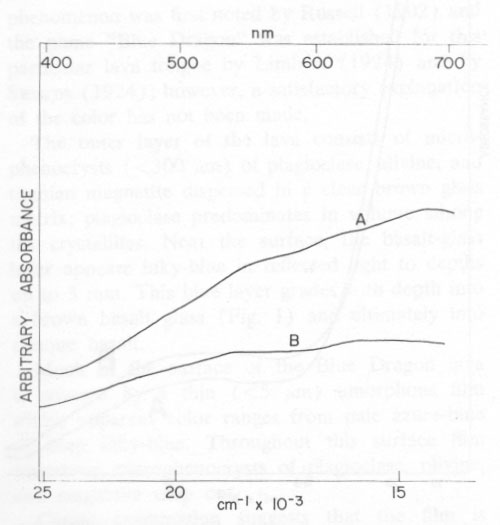
It is clear from Figure 2 why both glass samples are brown in transmitted light. The fact that spectrum A is less intense at ~ 1000 nm but more intense between 400 nm and 600 nm than spectrum B indicates that the glass from the blue layer is richer in Fe3+ than is the glass from the brown zone. (The total iron concentration is the same for both.) Thus, it seems that the outer layer of the lava was oxidized, and that the blue coloration associated with the magnetite particles in some way related to the degree of oxidation of this outer zone. Reflectance Spectra of Film and of the Blue Glass The diffuse reflectance spectrum A in Figure 3 is from freshly cleaned pieces of the lava, approximately half the surface area of which was accounted for by the thin amorphous film. To prepare the sample for spectral measurements, a wafer (~10 cm2) containing the surface film was cut from each of three pieces of basalt. Two of these pieces were cemented back to back, to expose the blue surface film of each. The resultant "sandwich" was cemented vertically on the blue surface of a third piece. This horizontal base-piece acted as a cap when the specimen was mounted in the integrating sphere of the reflectance accessory of a Cary 14 spectrophotometer. All surfaces of the three basalt slices that did not appear to be bright azure-blue to the unaided eye were painted with a flat white paint. For comparison, the diffuse reflectance spectrum (B in Figure 3) was made for a flat piece of basalt from which the siliceous film was ground away to expose the inky-blue glass layer. This spectrum was measured by simply placing the specimen over the sample port of the reflectance accessory. It is less intense than spectrum A because of the smaller area that was irradiated.
Both reflectance spectra in Figure 3 possess several absorption bands between 500 to 700 nm. These correlate with strong absorption of yellow to red light and with surface reflection of intense blue light. The similarity between the two spectra support the argument that the azure-blue color reflected by the Blue Dragon basalt originates from the inky-blue blotches surrounding the magnetite crystals in the gloss underlying the siliceous film. The siliceous film chiefly transmits and scatters this blue light. It is to be emphasized that spectra A and B are composites of light reflected from the magnetite particles and of brown light transmitted and/or reflected by the surface phenocrysts of plagioclase and olivine. Plagioclase crystals transmit brown light from the glass matrix because the concentration of blue-reflecting magnetite in their vicinity is relatively low. Possible Origin of the Blue Color The color and pleochroism of many silicates and other minerals are influenced by electron transfer between Fe2+ and Fe3+ ions, especially in structures where their coordination polyhedra share edges. In such cases a dominant absorption band in the 550 to 650-nm range of the visible spectrum results in a blue color. Examples are partly oxidized vivianite, certain sodic amphiboles (Hush, 1967; Faye, Manning, and Nickel, 1968; Faye and Nickel, 1970), and probably blue kyanite (Faye and Nickel, 1969). Similarly, Fe2+→ Ti4+ electron transfer is thought to be responsible for absorption bands between 550 and 700 nm in the spectrum of blue sapphire (Townsend, 1968; Ferguson and Fielding, 1971; Eigenmann and Gtinthard, 1972). Electron microprobe analysis of the magnetite particles indicates the presence of ~14 percent titanium, an unusually high value. Microprobe scanning and microscopic examination suggest the presence of a single phase rather than a micro-intergrowth of magnetite and ilmenite. Thus, on the basis of the high titanium content of the magnetite and the absorption envelope between 500 and 700 nm in the reflectance spectra in Figure 3, the electron transfer processes (Fe2+→ Fe3+ and Fe2+→Ti4+) may each contribute to the color of the Blue Dragon lava. As stated previously, the outer layer of the lava has been partly oxidized; it is proposed, therefore, that oxidation also has occurred at the surface of the titanian magnetite particles. If all the titanium in magnetite exists as Ti4+ (in the octahedral site), then the only effect of oxidation is to increase the concentration of Fe3+ - and thus decrease the number of interacting Fe2+ - Fe3+ and Fe2+ - Ti4+ pairs. Magnetite and similar iron-titanium spinels are considered to be black primarily because of intense absorption in the visible region caused by the collective interaction (energy bands) of Fe2+ and Fe3+ or of Fe2+ and Ti4+ ions located in octahedral sites. However, oxidation of Fe2+ could decrease the concentration of interacting cations to the extent that electron exchange becomes localized to adjacent pairs of ions (energy levels). This, in turn, might sufficiently decrease the intensity of the electron transfer bands centered in the 550-700 nm region until their high-energy wings, at least, "retreated" into the visible region to permit the reflection of blue light. It is difficult to determine the relative contribution of the Fe2+→ Fe3+- and the Fe2+ → Ti4+ electron transfer processes; however, because of the higher concentration of iron in the magnetite, the former should predominate over the latter. The principal absorption in the spectrum of blue sapphire is centered at approximately 570 nm, and is attributed to the Fe2+ → Ti4+ electron transfer process (Townsend, 1968; Ferguson and Fielding, 1971; Eigenmann and Günthard, 1972). Possibly this also holds for the spectra in Figure 3, and, if correct, the more intense: absorption between 600 and 700 nm in these spectra can then be assigned to the Fe2+ → Fe3+ electron transfer process. It is noteworthy that fractured surfaces of partly weathered magnetite that do not contain an appreciable concentration of titanium (i.e., <0.2 percent) are commonly seen to reflect blue to purple light. Although one of the authors (G.H.F.) has such a specimen, from Haliburton, Ontario, a satisfactory reflectance spectrum could not be obtained for comparative purposes. Summary The Blue Dragon lava seems to be unique in that its outer oxidized layer is strikingly blue in reflected light, but rich brown in transmitted light. Blue light, which reflects from the surface of small, partly oxidized, titanian magnetite particles, and which is caused by electron transfer between Fe2+ → Fe3+, and probably Fe2+ → Ti4+ pairs, may account for this phenomenon. This is another example of the importance of electron transfer processes in determining the optical properties of naturally occurring siliceous materials. However, the Blue Dragon lava is unusual in that the interacting species are not incorporated in a silicate lattice. Acknowledgments We thank Dr. D. C. Harris for electron microprobe analyses and advice, and Mr. R. G. Pinard for technical assistance. References EIGENMANN, K., AND Hs. H. GÜNTHARD (1972) Valence states, redox reactions and biparticle formation of Fe and Ti doped sapphire. Chem. Phys. Lett. 13, 58-61. FAYE, G. H. (1968) The optical absorption spectra of iron in six-coordinate sites in chlorite, biotite, phlogopite and vivianite. Some aspects of pleochroism in the sheet silicates. Can. Mineral. 9, 403-425. ________, P. G. MANNING, AND E. H. NICKEL (1968) The polarized optical absorption spectra of tourmaline, cordierite, chloritoid, and vivianite: ferrous-ferric electrons interaction as a source of pleochroism. Amer. Mineral. 53, 1174-1201. ________, AND E. H. NICKEL (1969) On the origin of colour and pleochroism of kyanite. Can. Mineral 10, 35-46. _________, AND ________ (1970) The effect of charge-transfer processes on the colour and pleochroism of amphiboles. Can. Mineral. 10, 616-635. FERGUSON, J. AND P. E. FIELDING (1971) The origins of the colours of yellow, green and blue sapphires. Chem. Phys. Lett. 10, 262-265. HUSH, N. S. (1967) Intervalence-transfer absorption. Part 2. Prog. Inorg. Chem. 8, 357. LEHMANN, G. (1970) Ligand field and charge transfer spectra of Fe(III)-O complexes. Z. Physik. Chem. N.F. 72, 279-297. LIMSERT, R. W. (1924) Among the craters of the moon. Nat. Geog. Mag. 45, 303-328. PAUL, A., AND R. W. DOUGLAS (1969) Optical absorption of trivalent iron in sodium borate glasses containing chlorite. Phys. Chem. Glasses, 10, 138-145. RUSSELL, I. C. (1902) Geology and water resources of the Snake River Plains in Idaho, U.S. Geol. Surv. Bull. 199, 1-192. STEARNS, H. T. (1924) Craters of the Moon National Monument. Geog. Rev. 14, 362-372. STEELE, F. N., AND R. W. DOUGLAS (1965) Some observations on the absorption of iron in silicate and borate glasses. Phys. Chem. Glasses, 6, 246-252. TOWNSEND, M. G. (1968) Visible charge transfer band in blue sapphire. Solid State Comm. 6, 81-83. Manuscript received, August 8, 1972; |