|
|
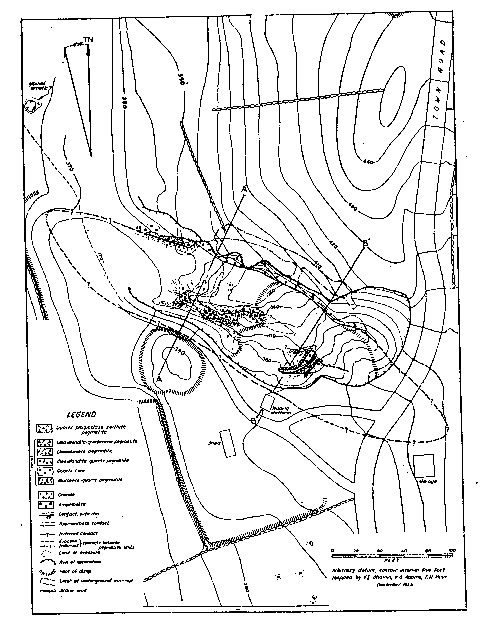
Volume 31, pages 329-345, 1946 VINCENT E. SHAININ ABSTRACT The Branchville pegmatite is a distinctly zoned body that has a complex history. In the first stage the following zones appear to have formed by successive deposition from the walls inward: border zone, muscovite-quartz zone, perthite zone, and quartz core. In the second stage, replacement bodies composed of cleavelandite-quartz, cleavelandite, and cleavelandite-spodumene were developed, at the expense of the zones, along the interzonal contacts. Books of sheet-bearing mica were replaced in parts of the muscovite-quartz zone and most of the microcline-perthite zone was replaced by cleavelandite and quartz. The third stage consisted of supergene alteration of previously formed minerals by circulating ground water. INTRODUCTION An unusual pegmatite in the village of Branchville, Connecticut, has long been a renowned locality for manganese phosphates as well as a source of commercial minerals. Nine minerals were first described from the pegmatite, and quartz, feldspar, mica, spodumene, beryl and columbite have been mined there at various times. The demand for sheet mica during World War II induced operators to work the long dormant mine in 1943 and 1944. Unwatering and underground mining permitted detailed studies of the geology by the writer in 1944 as part of the strategic-minerals investigations of the United States Geological Survey. Although studies of some minerals in the pegmatite have been made by Boltwood (2), Brush and Dana (3, 4, 5, 6, 7), Comstock (10, 11), Hawes (13), Hillebrand (16), Penfield (19, 20), von Rath (24), Osborne (18), and Wright (25), no account of its internal structure or economic geology has been published. Brush and Dana commented on the paragenesis of the phosphates, but no paragenetic study of the pegmatite as a whole has previously been made. The mine lies 550 feet N. 48° E. of the railroad station in Branchville. To reach the village from White Plains, New York, travel 22 miles eastward on the Merritt Parkway to route U. S. 7, then proceed 9 miles northward on route 7 to Branchville. The railroad station lies on the eastern side of the highway, on the Housatonic Branch of the New York, New Haven and Hartford Railroad. The first excavation in the pegmatite was made about 1876 by A. N. Fillow, then owner of the property. He is reported to have mined mica. The mica recovered was then considered of inferior quality, and operations ceased sometime before the spring of 1878. At that time, George J. Brush and Edward S. Dana, both of Yale University, became so enthused about the new minerals at Branchville that they engaged Fillow to excavate the deposit with funds furnished by Yale. In 1880 the Union Porcelain Works of Greenpoint, New York, bought the property and operated it for feldspar and quartz until at least 1890. The Bridgeport Wood Finishing Company of Bridgeport, Connecticut, is said to have operated for quartz and feldspar prior to 1920. From September 1943 to November 1944, Fred and Joseph Burrone and Carlo Rusconi, all of North Branford, Connecticut, operated the mine for mica, and the Sandy Ridge Mica and Mining Company, Inc., 927 15th Street N. W., Washington, D. C., worked the mine in November and December 1944. The main working is an open cut 240 feet long, 50 to 85 feet wide and 60 feet in maximum depth. A crosscut 20 feet long has been driven into the northern wall of the cut. From the crosscut one drift extends 75 feet northwest, and another 57 feet southeast. Both open cut and drifts are partly backfilled. ACKNOWLEDGMENTS Before the final draft of this paper could be prepared the writer was called abroad, and his friend, Eugene N. Cameron, Commodity Geologist for Industrial Minerals, U. S. Geological Survey, therefore assumed the task of editing and revising the paper. The writer is deeply indebted to Dr. Cameron for this favor and also to Prof. Andrew H. McNair, Dartmouth College, for his appreciated criticisms. Drs. George Switzer and Harold Mikami, Yale University, generously rendered valuable assistance in identifying minerals collected by the writer. Special thanks are due to Prof. Glenn W. Stewart, University of New Hampshire, for his aid in determining refractive indices of feldspar, and to Benjamin Levin, U. S. Bureau of Mines, who gave valuable advice during the field work. Frederic Main and Karl Adams, U. S. Geological Survey, aided in mapping, and the operators of the Branchville mine also cooperated generously and wholeheartedly. STRUCTURE AND COUNTRY ROCK The pegmatite appears to be tabular, but only its hanging wall is exposed (Fig. 1). It is at least 200 feet long and at least 40 feet thick. The hanging wall strikes north 60 degrees west and has an average dip of 45 degrees northeast. Two features suggest that the body either plunges beneath country rock in both directions along strike or terminates. These are: (1) the presence of country rock outcrops to the southeast on strike with the pegmatite, and (2) the lack of outcrops of the pegmatite outside of the workings. The pegmatite probably terminated up dip a short distance above the quarry.
FIG. 1. Geologic map of the Branchville Mica Mine, Redding, Connecticut.
The country rock is a medium-grained (2-4 mm.) dark brownish-green amphibolite composed of hornblende, biotite, and plagioclase with accessory sphene and ilmenite. The rock was mapped by Gregory and, Robinson (12) as part of the Danbury granodiorite gneiss. The amphibolite contains a few small, angular inclusions of fine-grained (0.2-0.3 mm.) gneiss composed of plagioclase, biotite, and quartz. In the north western corner of the quarry a granite dike, 10 feet thick, cuts the amphibolite and in turn is cut by the pegmatite. The dike is a gray, medium-grained gneissoid biotite-muscovite granite, containing accessory apatite, magnetite and zircon. Numerous small angular xenoliths of amphibolite occur in the dike. According to Agar (1), this and other granite dikes in the vicinity are part of the Thomaston granite, which is probably late Ordovician in age. The western margin of a stock of Thomaston granite lies 500 feet northeast of the mine. The stock crops out over an area 3½ miles long and l½ miles wide, and is probably the intrusive from which the Branchville pegmatite was derived. Shaub (23) averaged 5 analyses of uraninite from the pegmatite and concluded that the age of the mineral is 368 million years, or probably late Ordovician. |
|
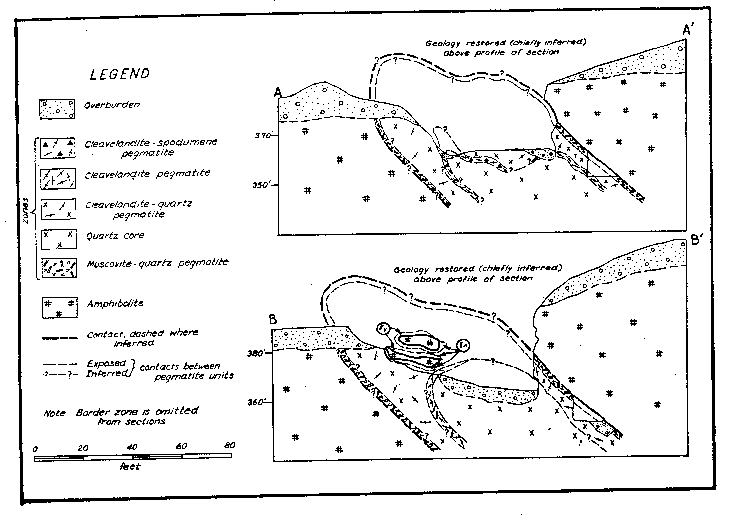
Fig. 2. Structure sections, Branchville Mica Mine, Redding, Connecticut INTERNAL STRUCTURE AND COMPOSITION OF THE PEGMATITE General The pegmatite is composed chiefly of quartz and cleavelandite with subordinate muscovite. It has a striking internal structure. The following lithologic and structural units are found successively inward from the wall: border zone, muscovite-quartz zone, cleavelandite-quartz unit, cleavelandite unit, cleavelandite-spodumene unit and quartz core (Figs. 1 and 2).1 These units are bodies of rock that differ in texture or mineralogy, or both. Border zone The border zone, 2 to 6 inches thick, is composed of one-quarter inch grains of quartz and oligoclase (An 14) with subordinate muscovite and accessory green apatite, green tourmaline, hornblende, and biotite. Calcite is a minor constituent. In some places the zone consists largely of quartz, and oligoclase is lacking; in others oligoclase is the only essential mineral. Hornblende and biotite occur in the upper part of the zone and are visible only in thin section. They were probably derived from the amphibolite. Calcite occurs in narrow veins, commonly along the contact with amphibolite. Muscovite-quartz zone The muscovite-quartz zone (or wall zone) is 1 to 2½ feet thick. Its average thickness is 1½ feet. It is composed of muscovite, quartz, and cleavelandite (An 5-6) with minor apatite, pyrite, tourmaline and fluorite. Muscovite constitutes 30-60% of the zone and occurs in deep rum-colored books oriented with cleavage roughly perpendicular to the hanging wall (Fig. 3). The books, easily broken from their matrix, range from 1 to 24 inches in diameter and 1/8 to 12 inches in thickness. Most of the books are 5 inches in diameter and 1 inch thick. Ruling is a common defect. Cross-fracturing, A-structure and reeving are present in some books. Most of the mica is hard and free-splitting.
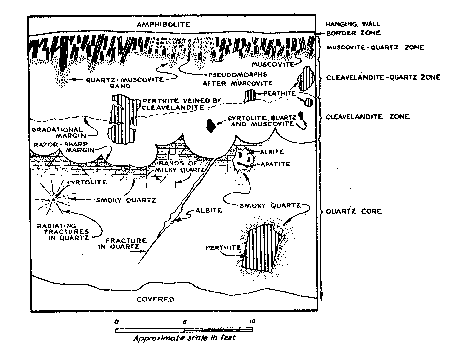
FIG. 3. Composite sketch of northeastern quarry face showing mutual relations between minerals and zones. Scale of small features is slightly exaggerated.
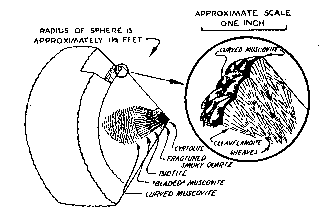
Approximately 15 percent of the muscovite visible in the wall zone in December 1944 had been replaced by material that is too fine-grained to identify in thin section, but which appears to be albite (An ca. 5) and quartz. Perfect pseudomorphs after muscovite have been formed. The replacement has been limited to five parts of the zone. The three largest parts occur in the eastern third of the workings, and are irregular in outline. Their distribution does not appear to be influenced by structural or mineralogical control. Within them practically all of the muscovite adjacent to the wall-rock contact, including that in the border zone, has been replaced. Most of the mica in the lower 1/3 or 1/4 of the wall zone is unaffected by replacement. Large books, occupying the full thickness of the zone, grade from unaltered muscovite in the lower part to pseudomorphs in the upper part of the zone (Fig. 3). The margin between replaced and unreplaced portions of a single book is gradational over not more than 4 inch. In thin section, small, scattered remnants of muscovite are visible in the replaced portions. It seems likely that replacement of muscovite began at the wall-rock contact and advanced toward the center of the pegmatite. Although the footwall of the pegmatite is not visible, the wall zone may be developed along it. The evidence for this is found around two large bodies of amphibolite that are probably xenoliths in the pegmatite (section B-B'). The border and wall zones of the pegmatite surround each of the bodies. Studies by the writer of other pegmatites in Connecticut (Toll Gate mine, Middletown; Cramer mine, Portland; Gotta Walden mine, Portland) have suggested that a xenolith bounded on its lower surface by a wall zone may indicate the presence of a similar zone on the hanging wall of the pegmatite. Likewise (as in the Cramer mine) a xenolith bounded on both surfaces by a wall zone may indicate the presence of that zone along both walls of the pegmatite. At Branchville, the mica-bearing wall zone is present on both upper and lower surfaces of the amphibolite bodies. It is believed likely, therefore, that the zone is present adjacent to the unexposed footwall. Cleavelandite-quartz unit The cleavelandite-quartz unit has an average thickness of three feet, and is composed of cleavelandite and quartz with subordinate muscovite. Minor constituents are perthite and garnet. Cleavelandite is commonly white, fine-grained (laths 1/8-1/4 inch long), and compact. Muscovite occurs in pale-green books 1/2-1 inch in diameter. Concentrically curved books are rare in this unit, although they are common in the cleavelandite unit. Perthite occurs in scattered anhedral crystals, 1 to 5 feet long, that vary from grayish-white to light buff-brown. One crystal, 3 feet long, is partly in the cleavelandite-quartz unit and partly in the cleavelandite unit. The crystal is veined throughout its length by minerals of both zones (cleavelandite, quartz, garnet and muscovite) (Fig. 3). This, and other crystals of microcline-perthite in the cleavelandite-quartz unit and cleavelandite unit, appear to be unreplaced parts of a pre-existing microcline-perthite zone. Cavities, 1/2 to 4 inches in diameter, occur in the zone. Superposition of one mineral on the crystal faces of another is common in these cavities. Albite (An 2-3) is found in most of the cavities and commonly forms the lining on which the following minerals have grown, in the order listed: perthite, quartz, muscovite, apatite, calcite, and pyrite. Perthite occurs in reddish pink subhedra 1/4 inch long. Albite is a very minor constituent in these crystals compared with the coarser perthite mentioned above. Apatite, in colorless, white, pale-yellow, or purple prisms, occurs on crystals of all the other minerals, but its crystal faces rarely support other minerals. Small euhedral crystals of albite and green muscovite are often partially embedded in quartz crystal faces, indicating an overlapping sequence of crystallization. Cleavelandite unit The cleavelandite unit is a discontinuous body lying along the core of the pegmatite. For the most part it ranges from 2 to 2½ feet in thickness, and is composed of cleavelandite, with subordinate muscovite, cyrtolite, quartz, and biotite. Apatite, garnet, perthite, columbite-tantalite, and other minerals are minor constituents. The unit has an unusual structure. It consists of laterally coalescing hemispheres that commonly have a radius of 1 to l½ feet. The outer margin of the unit grades indistinctly into the cleavelandite-quartz unit and the inner margin has a very sharp contact with the quartz core. Fig. 4. Sketch of section through a hemisphere in the cleavelandite zone. Inset in circle is an enlargement showing the relation between cleavelandite sheaves and books of curved muscovite.
Each hemisphere in the unit may be considered as a separate structural element (Fig. 4). Four minerals occur invariably in a regular succession. One or more of these minerals may be absent in a given hemisphere, but if so, the order of the others does not change. Commonly at the center of the hemisphere is a brown crystalline aggregate of cyrtolite, 1 inch in diameter that shows numerous tetragonal pyramids with curved faces. In some hemispheres small masses of smoky quartz, 1 to 6 inches in diameter, lie adjacent to one or more sides of the cyrtolite aggregate. The quartz is invariably shattered by fractures radiating outward from the cyrtolite. In a few hemispheres, the nucleus is a mass of light-smoky quartz, 1 to 4 inches long, which is a component part of the cleavelandite-quartz zone. Outside the quartz lies brown biotite, usually in narrow blades (½ to 1 inch long) in which the cleavage is parallel to radii of the hemisphere. The biotite passes outward into similarly oriented blades of greenish muscovite (½ to 3 inches long). Outside the muscovite blades are concentrically curved muscovite books whose cleavage is subparallel to the surface of the hemisphere. The innermost curved books are often less than 3 mm. in diameter; they gradually increase in size toward the surface of the hemisphere, where they are usually ½ to 1 inch broad. The transition from bladed to curved books is sharp and distinct. Biotite and both types of muscovite, in most cases, have the form of a cigar-shaped body whose long axis is parallel to a radius of the hemisphere (Fig. 4). The curved muscovite at the distal termination of such a cigar-shaped body, may or may not lie on the surface of the hemisphere. Often the curved books form unbroken spherical shells separated from one another by thicker intervening shells of cleavelandite. Cleavelandite in the hemispheres occurs in fine-grained white laths, often curved, which are always grouped in radiating sheaves, commonly 1 inch in length. The rounded upper surface of each sheaf is commonly smooth and hemispherical. In the ideal case the sheaves are oriented with their curved surfaces tangent to the surface of the main hemisphere of which they are a part. The lateral coalescence of hundreds of these small sheaves forms the main hemisphere, the surface of which has an uneven, cobblestone-like appearance. The curvature of the muscovite conforms to the rounded tops of the cleavelandite sheaves, and in simple cases, each book lies on the curved surface of a sheaf (Fig. 4). This strongly suggests that the muscovite is either contemporaneous with or later than the cleavelandite. In the aggregates of curved muscovite forming part of the cigar-shaped bodies mentioned above, the books lie on top of one another and their basal cleavages are nearly parallel. The first books to form may have been contemporaneous with formation of the sheaves, or they may have formed by replacement of the rounded surfaces of the sheaves. Later in the sequence muscovite may have replaced the remaining cleavelandite and the curvature of these books may have been inherited from the adjoining, previously formed books.
The manganese phosphates, of which lithiophilite is the most common, occur in rare, scattered concentrations within the cleavelandite-spodumene unit. Yellowish-brown lithiophilite occurs in isolated, ellipsoidal nodules from 1/4 inch to more than one foot long; their average length is 3 inches. The nodules are invariably coated with bluish-black manganese oxide. In some specimens, lithiophilite veins cut spodumene indicating later crystallization of the phosphate. Numerous veins of cleavelandite cutting lithiophilite indicate that the silicate continued to form after the phosphate.
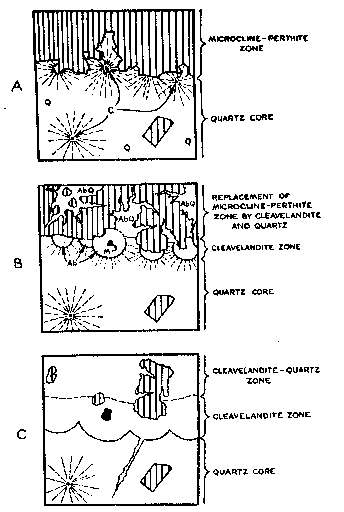
Crystalline cyrtolite aggregates in the core, similar to those in the cleavelandite unit, are completely surrounded by a halo of smoky quartz, usually 2 feet in diameter. Conspicuous fractures in the quartz radiate outward in all directions from the cyrtolite nucleus. The zone of fracturing coincides roughly with the extent of the halo. A similar occurrence has been described by Landes (17) from the Baringer Hill, Texas, pegmatite where rare earths occur in massive quartz which is radially shattered, forming so-called "stars" 8 to 10 feet in diameter. Hidden (15) found that the "stars" at Baringer Hill served as clues to rare minerals at their center. Hess and Wells (14) believed that the growth of the nuclear mineral "took place faster than the older mineral is replaced, breaking the rock radially," and Landes has concurred in this opinion. ORIGIN OF THE PEGMATITE General Three stages in the formation of the pegmatite are postulated: a first stage during which the zones developed; a second stage marked by partial replacement of the zones; and a third stage of supergene alteration. There is no evidence at Branchville to indicate the order in which the border zone, muscovite-quartz zone, microcline-perthite zone and quartz core formed. However, their relationships to the cleavelandite-quartz, cleavelandite, and cleavelandite-spodumene units indicate that the zones developed at an earlier stage, and as there is no evidence that the zones were developed at the expense of pre-existing massive pegmatite, zone development appears to represent the first stage in the development of the pegmatite as a whole. During the first stage, there may have been limited replacement of earlier formed minerals by those later in the sequence. Near the end of, or after, the first stage, the evidence suggests that hydrothermal solutions altered the zones, particularly those with abundant minerals, such as perthite, susceptible to replacement. New units were developed along the contacts between the zones because the zones provided favorable loci. Some solutions followed the wall rock contact of the pegmatite and replaced parts of the border and wall zones. The third stage consisted of supergene alteration of previously formed minerals by circulating ground water. In both first and second stages, plagioclase grew progressively more sodic. Indices of refraction, determined by the single variation method, indicate anorthite contents as follows: border zone, An 14; muscovite-quartz zone, An 5-6; cleavelandite-quartz unit, An 3; cleavelandite unit, An 3; subhedra in cavities in the cleavelandite-quartz unit, An 2-3. There was no detectable difference between specimens from the same zone or unit collected over a distance of almost 200 feet along strike. First stage In the first stage, the border zone, muscovite-quartz zone, microcline-perthite zone and quartz core were formed. From analogy to other New England pegmatites (8) it seems likely that the order of the zones from the walls inward is likewise the order in which they were deposited, but no positive evidence of this has been found at Branchville. The microcline-perthite zone has been almost completely obliterated by hydrothermal replacement. Microcline-perthite anhedra, which occur throughout the cleavelandite and cleavelandite-quartz units, are clearly veined by cleavelandite, quartz, muscovite and garnet. This indicates the presence in both units of microcline-perthite earlier in age than the most abundant constituents of the units. A microcline-perthite zone-of the kind postulated at Branchville - surrounding a quartz, or quartz-rich core is a common feature in other New England pegmatites that have suffered little or no hydrothermal replacement (9, 21). Although the present visible margin of the core is exceedingly sharp and well defined (as a result of the replacement postulated below), the original boundary between the microcline-perthite zone and the core was probably similar to that in other New England pegmatites (9), possessing cores of primary origin. The margins are commonly gradational in that some feldspar subhedra project into the core or lie wholly within the core near its margins, but it is also sharp with respect to the contacts between individual feldspar crystal faces and the quartz (Fig. 5). This distinction is vital to an appreciation of the influence exerted by the margin of the core over the passage of later solutions. Second stage The second stage appears to have been complex. Two phases are discernible: an early phase during which the bulk of replacement occurred, and a late phase during which cavity fillings were deposited. The cleavelandite-quartz, cleavelandite, and cleavelandite-spodumene units formed during the second stage. The sequence of formation is not known. The chief loci of replacement were the perthite zone and the margin of the core. By far the most abundant mineral formed in this stage was cleavelandite. Quartz, muscovite, garnet, spodumene, the manganese phosphates and many rare minerals also formed at this stage. Many of the unusual structures of the cleavelandite unit are best explained by replacement in this stage. The formation, in the core, of cyrtolite aggregates which radially shattered the surrounding quartz, apparently preceded formation of the cleavelandite unit. Sharp boundaries between crystal faces of perthite subhedra (on the inner margin of the perthite zone) and quartz of the core are believed to have provided passageways for hydrothermal solutions rich in soda and silica. Aided by the radial fractures these solutions gained access to cyrtolite aggregates lying close to the core's margin, and biotite, muscovite and cleavelandite began to crystallize around the cyrtolite. It seems likely that the system of radial fractures emanating from the cyrtolite was responsible for the spherical form assumed by the aggregate of newly formed minerals. Small bodies of radially shattered smoky quartz are sometimes present adjacent to cyrtolite nuclei (Fig. 4) within the hemispheres. These quartz bodies appear to be unreplaced parts of the quartz core. The lateral coalescence of neighboring hemispheres gave rise to the cleavelandite unit. Probably contemporaneous with formation of the cleavelandite unit vas the introduction of albite in the core as fracture fillings, replacement veins and isolated "unsupported" aggregates.
FIG. 5. Idealized sketches showing postulated origin of cleavelandite-quartz zone and cleavelandite zone. (Q=quartz; AbQ=cleavelandite and quartz; Ab=cleavelandite; M= aggregate of curved muscovite; C=cyrtolite surrounded by radiating fractures in quartz; shaded parts = predominant microcline-perthite.)
No evidence was observed to indicate the paragenetic sequence of most of the minerals of the cleavelandite-spodumene unit. Minerals of the late phase occur as crystals lining cavities. Carbonate and sulfide minerals are supported by the crystal faces of earlier minerals and were last to form in the cavities. Calcite veins in the border zone probably formed at this time. Supergene alteration. Supergene alteration resulted in the formation of manganese oxide and purpurite from lithiophilite, and limonite from pyrite. CONCLUSIONS General The Branchville pegmatite is a distinctly zoned body with a complex history. A first stage is recognizable in which the following zones formed, possibly from the walls inward by successive deposition: border zone, muscovite-quartz zone, perthite zone and quartz core. Replacement in a second stage altered the zones, and created new units along the contacts between the zones. The configuration of the units of the second stage therefore reflects the form and attitude of the zones because of the favorable loci provided by these pre-existing units. The cleavelandite-quartz unit, cleavelandite unit, and cleavelandite-spodumene unit are, believed to have formed in the secondary stage. The third stage consisted of supergene alteration of previously formed minerals by circulating ground water. There is no evidence to indicate termination of the sheet-mica bearing zone either down dip or along strike. However, a factor which should be studied with extreme caution in future operations for mica is replacement of muscovite in the zone. Whether or not the distribution of replaced portions is influenced by discernible geological factors needs determination. Studies to date have failed to reveal the presence of any controlling factor. It is anticipated that the quartz core continues as a virtually pure body down dip, and it is quite probable that several thousand tons of hand-separable quartz is available. There is no evidence to indicate the presence of a sizeable deposit of potash feldspar down the dip. However, if the crest of the core is present beneath deep backfill in the southeastern end of the quarry, some feldspar may lie directly above the crest. Although a small reserve of spodumene is present in the pegmatite, a large percentage of the crystals are more or less altered. In addition, difficulty might be encountered in hand-cobbing spodumene crystals because of their small average size. It is unlikely that the pegmatite contains sufficient quantities of beryl, columbite-tantalite, uraninite and other rare minerals of economic importance to warrant commercial exploitation for them. However, one or more may be recovered as byproducts from operations not centered on the wall zone. Replacement deposits that transect zonal structure have been recognized before in pegmatites. However, the occurrence of replacement bodies, that do not, in general, transect earlier-formed zones has not been widely recognized. The evidence at Branchville suggests that such replacement bodies can easily be mistaken for zones unless carefully studied. NOTES 1 The classification of pegmatite units followed is that developed jointly by geologists of the Federal Survey engaged in investigations of domestic pegmatites during the war. REFERENCES 1. AGAR, W. M., The granites and related intrusives of western Connecticut: Am. Jour. Sci., 27, 373 (1934). 2. BOLTWOOD, B. B., On the ultimate disintegration products of the radioactive elements: Am. Jour. Sci., 4th ser., 23, 78-88 (1907). 3. BRUSH, G. B., AND DANA, E. S., On a new and remarkable mineral locality in Fairfield County, Connecticut: Am. Jour. Sci., 3d ser., 16, 33-46 (1878). 4. BRUSH, G. B., AND DANA, E. S., On the mineral locality in Fairfield County, Connecticut, with description of two additional new species: Am. Jour. Sci., 3d ser., 17, 359-368 (1879). 5. BRUSH, G. B., AND DANA, E. S., On the mineral locality in Fairfield County, Connecticut: Am. Jour. Sci., 3d ser., 18, 45-50 (1879). 6. BRUSH, G. B., AND DANA, E. S., On the mineral locality at Branchville, Connecticut. Fourth paper. Spodumene and the results of its alteration: Am. Jour. Sci., 3d ser., 20, 257-284 (1880). 7. BRUSH, G. B., AND DANA, E. S., On the mineral locality at Branchville, Connecticut. Fifth paper: Am. Jour. Sci., 3d ser., 39, 201-216 (1890). 8. CAMERON, E. N., LARRABEE, D. M., MCNAIR, A. H., PAGE, J. J., SHAININ, V. E., AND STEWART, G. W., Structural and economic characteristics of New England mica, deposits: Econ. Geol., 40, 369-393 (1945). 9. CAMERON, E. N., LARRABEE, D. M., MCNAIR, A. H., PAGE, J. J., SHAININ, V. E., AND STEWART, G. W., U. S. Geol. Survey report on New England pegmatites, in preparation. 10. COMSTOCK, W. J., Analyses of some American tantalates: Am. Jour. Sci., 3d ser., 19, 131-132 (1880). 11. COMSTOCK, W. J., On the chemical composition of the uraninite from Branchville, Connecticut: Am. Jour. Sci., 3d ser., 19, 220-222 (1880). 12. GREGORY, H. E., AND ROBINSON, H. H., Preliminary geological map of Connecticut: Conn. Geol. and Nat. Hist. Survey, Bull. 6, 84 (1906). 13. HAWES, G. W., On liquid carbon dioxide in smoky quartz: Am. Jour. Sci., 3d ser., 21, 203-209 (1881). 4. 14. HESS, F. L., AND WELLS, R. C., Samarskite from Petaca, N. M.: Am. Jour. Sci., 19, 21 (1930). 15. HIDDEN, W. E., Some results of late mineral research in Llano County, Texas: Am.Jour. Sci., 4th ser., 19, 427 (1905). 16. HILLEBRAND, W. F., On the occurrence of nitrogen in uraninite and on the composition of uraninite in general: Am. Jour. Sci., 3d ser., 40, 384-394 (1890). Also in U. S. Geol. Survey Bull. 78, 64 (1891). 17. LANDES, K. K., The Baringer Hill, Texas, pegmatite: Am. Mineral., 17, no. 8, 381-390 (1932). 18. OSBORNE, T. B., The qualitative determination of niobium: Am. Jour. Sci., 3d ser., 30, 336 (1885). 19. PENFIELD, S. L., Analyses of two varieties of lithiophilite (manganese triphylite) : Am. Jour. Sci., 3d ser., 26, 176 (1883). 20. PENFIELD, S. L., Effect of the mutual replacement of manganese and iron on the optical properties of lithiophilite and triphylite: Am. Jour. Sci., 3d ser., 50, 387-390 (1895). 21. SHAININ, V. E., Pegmatites of Topsham, Maine, report in preparation. 22. SCHAIRER, J: F., Minerals of Connecticut: Connecticut Geol, and Nat. Hist. Survey, Bull. 51, 100, 101 (1931). 23. SHAUB, B. M., The occurrence, crystal habit, and composition of the uraninite from Ruggles Mine, near Grafton Center, New Hampshire: Am Mineral., 23, no. 5, 339 (1938). 29. VON RATH, GERHARD, Mineralien aus den Vereinigten Staaten : Niederrhein Ges. Bonn, Szb. 42, 56-62 (1885). 25. WRIGHT, A. W., On the gaseous substances contained in the smoky quartz of Branchville, Connecticut: Am. Jour. Sci., 3d ser., 21, 209-216 (1881). NOTES * Published by permission of the Director, U. S. Geological Survey. [var:'startyear'='1946'] [Include:'footer.htm'] |