|
|
Volume 24, pages 791-809, 1939 GRANITE PEGMATITES OF THE MT. ANTERO REGION, COLORADO GEORGE SWITZER, Harvard University, Cambridge, Mass. ABSTRACT A granite stock in the region of Mt. Antero, Chaffee County, Colorado, contains numerous, small, closely associated beryllium-rich pegmatites and veins. The pegmatites have the typical magmatic minerals microcline and quartz, and a variety of later hydrothermal minerals -beryl, phenakite, albite, bertrandite and fluorite. The veins are shown to be equivalent to the hydrothermal phase of the pegmatites. The upper temperature limit of formation of the pegmatites is approximately 600°C., as shown by frequent development of trigonal trapezohedron faces on smoky quartz crystals. The lower temperature limit of crystallization of the vein minerals is less than 200°C., as indicated by the position of adularia in the sequence of mineralization. A new angle table has been calculated for bertrandite. Twinned octahedra of fluorite are described. The relation between the structure and morphology of phenakite is pointed out, and the crystallographic elements of phenakite recalculated in a new, preferred setting. INTRODUCTION The region of Mt. Antero, Colorado has been known for many years as a locality for fine crystallized specimens of beryl (aquamarine), phenakite and bertrandite. Descriptive notes on these minerals have been published by Cross (1887), Smith (1887), Penfield (1887, 1888, 1890), Penfield and Sperry (1888), Over (1928; 1935), Pough (1935, 1936), and Montgomery (1938). However, no detailed study has been made of the occurrence of these minerals. For this purpose the writer spent six weeks during the summer of 1938 in the Mt. Antero region, in company with Mr. Arthur Montgomery of New York City, and Mr. Edwin Over of Colorado Springs, Colorado. The writer wishes to acknowledge his indebtedness to Professor Charles Palache, who suggested this study and made it possible by a grant for field expenses from the Holden Fund of the Department of Mineralogy of Harvard University. The cooperation of Messrs. Montgomery and Over, who spent their 1938 collecting season in the Mt. Antero region, was greatly appreciated. LOCATION AND GENERAL GEOLOGY The pegmatites and veins of the Mt. Antero region are almost entirely confined to a granite stock which is roughly elliptical in outline and approximately three miles across. The summits of Mt. Antero (14,245 ft:) and White Mountain (13,900 ft.) lie within the stock. These two mountain peaks are near the southern end of the Sawatch Range of the Southern Rocky Mountains and are about 15 miles northwest of Salida, Chaffee County, Colorado |
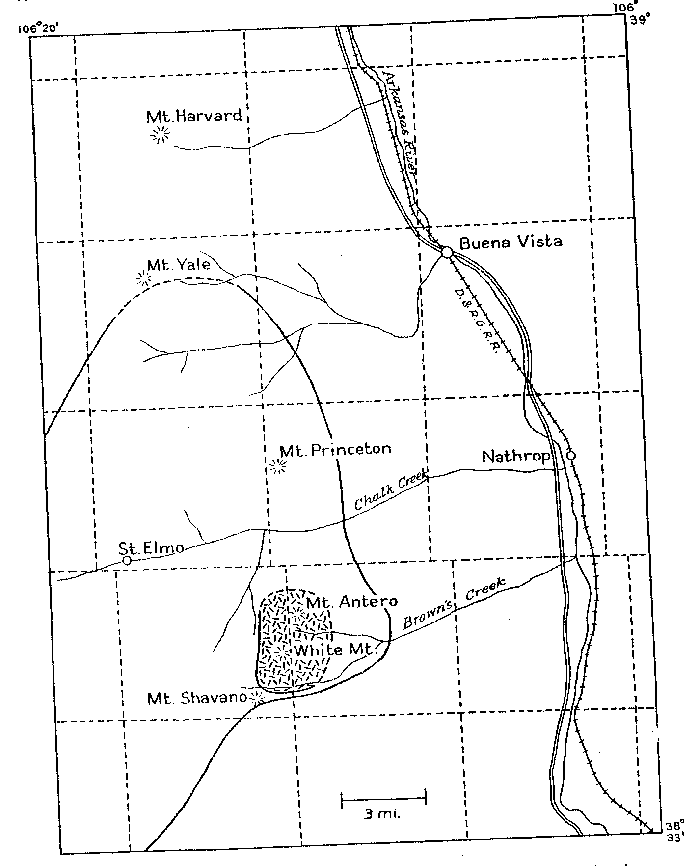
| FIG. l. Map showing location and geologic setting of Mt. Antero, Colorado. The granite stock is part of a much larger batholith of quartz monzonite, called by Crawford (1924) the Princeton batholith, and dated by him as being late Mesozoic or Tertiary in age. On the map, Fig. 1, the boundary of the Princeton batholith is shown as given by Crawford, and the smaller hatched area represents the granite stock in which the pegmatites and veins are found. The quartz monzonite is typically rather dark in color, of medium to coarse grain, and composed of subhedral andesine and green hornblende, anhedral orthoclase, quartz and biotite. The minor accessories are sphene, apatite and iron oxides. The granite is medium grained, and composed of subhedral oligoclase, anhedral orthoclase and quartz. The mafic minerals, green hornblende and biotite, are present in only small amount. Numerous fine-grained aplite dikes cut the granite and quartz monzonite. The minerals that have been described from Mt. Antero are found both in pegmatites and veins. All of the pegmatites seen were within the granite stock. The veins are in both granite and quartz monzonite. PEGMATITES AND VEINS The pegmatites are uniformly small in size, seldom exceeding 3 feet in width and extending laterally for only a few feet. Clean-cut exposures are few because of extreme frost action. All of the pegmatites and veins are found well above the timber line, in an area entirely covered with a mantle several feet deep of disintegrated rock. However, several outcroppings in vertical cliffs showed the pegmatites to be isolated lenticular bodies having sharp contacts with the granite enclosing them. Complete excavation of these pegmatites revealed that they are roughly disk-shaped or cylindrical bodies of limited extent in all directions. About 15 pegmatites and veins were studied in detail. The distinction between-pegmatite and vein is made chiefly on the basis of mineralogy, the differences in mineralogy being taken as a direct connotation of the physico-chemical conditions of deposition. On the same basis the pegmatites and veins have been further subdivided. This subdivision is done not only to facilitate description but to, point out that, although all of the pegmatites and veins arose from a common source, there are significant differences in their paragenesis, which will be discussed in a later section of the paper. The pegmatites and veins have been divided into the following types: A. Pegmatites 1. Beryl pegmatites (a) Beryl-smoky quartz 2. Phenakite pegmatites (a) Phenakite-colorless quartz (b) Phenakite-smoky quartz 3. Beryl-phenakite-bertrandite pegmatites (a) Beryl-phenakite-bertrandite (b) Beryl-phenakite-bertrandite-fluorite 4. Topaz pegmatites B. Veins 1. Muscovite-quartz vein 2. Phenakite-quartz-fluorite vein 3. Beryl-quartz-molybdenite vein
BERYL PEGMATITES Pegmatites characterized by beryl are the most abundant of the complex pegmatites of the region. The beryl pegmatites are of two types, those that have "pockets" and those that do not. The pegmatites having no pockets are small dikes or lenses of common pegmatite, with some subhedral beryl, but with no open-space deposition. The pegmatites containing pockets have been further complicated by successive deposition of fine euhedral crystals of microcline, beryl, smoky quartz, albite and fluorite on the walls of the pockets. Beryl-smoky quartz pegmatites: Several pegmatites of this type which had, pockets containing a variety of well crystallized minerals were found. One of the best exposed of these pegmatites was found by Over, near the summit of White Mountain. It out-cropped on a vertical cliff, and, therefore, was one of the least weathered of all the pegmatites seen. The pegmatite was a lens-shaped body approximately 12 inches across and had a roughly cylindrical central cavity extending back about 4 feet into the cliff. The pocket minerals were attached to the walls of the central opening and projected into it. The open, space was partially filled with "pocket dirt," most of which was formed by surface weathering. The periphery of the pegmatite was graphic granite several centimeters thick, to which was attached the pocket minerals. Fine crystals of perthitic microcline ranging up to 10 cm. in length were found both as single individuals and as Carlsbad and Baveno twins. Beryl started to crystallize at the close of the microcline deposition. The beryl crystallized in fine green to deep blue transparent crystals up to 7 cm. in length. Smoky quartz followed beryl, and formed sharp crystals up to 10 cm. in length and of deep smoky color. The smoky quartz formed as low-quartz, as shown by frequent development of faces of a trigonal trapezohedron. Muscovite followed quartz and in part was deposited contemporaneously with it. Albite closely followed muscovite and can be seen replacing muscovite. However, some of the muscovite is definitely later than the albite. It is not known whether this represents continuous deposition of muscovite, or two separate generations. Albite in some cases formed complete replacement pseudomorphs of microcline; in others it only partially replaced it, or formed shells about the microcline crystals. Most of the microcline was then removed by solution, leaving hollow shells of albite. Abundant sericite was formed by the alteration of microcline. Fluorite, colorless quartz and limonite were present in small amount. A tabular summary of the paragenesis of this pegmatite is given in Table 1, column 1. PHENAKITE PEGMATITES Next in abundance to pegmatites characterized by the presence of beryl are those which have only phenakite as a beryllium mineral, and show no evidence of beryl having been present. All of the pegmatites of this type contain pockets, but the complexity of the pocket-mineralization is greater where smoky quartz is present. Here, as in the beryl pegmatites just described, the presence of a cavity containing smoky quartz seems to indicate a center of more prolonged mineralization, with gradually decreasing temperature. Phenakite-colorless quartz pegmatite. The simplest of the phenakite pegmatites are in a pegmatitic area exposed on a cliff on the southeast side of Mt. Antero. The pegmatites are highly siliceous, being composed dominantly of milky quartz, with some subhedral microcline. The phenakite is found in small pockets lined with crystals of colorless or light smoky quartz ranging up to several centimeters in length. The phenakite crystals are both single and twinned and have a maximum length of about 5 mm. They are found either loose in the pockets or partially embedded in quartz. The only other mineral present is a small amount of muscovite. Phenakite-smoky quartz pegmatite. In the same pegmatitic area just described one smoky quartz pocket was found by Over. The pocket was approximately 18 inches across and 4 feet deep, and was lined with large crystals of microcline and smoky quartz. Euhedral crystals of microcline with a maximum dimension of 22 cm. show an etched peripheral zone about 2 cm. wide formed by the solution of the albite lamellae of the perthite. Muscovite was not abundant and in part overlapped with microcline in its deposition. Smoky quartz was very abundant and formed crystals ranging up to 30 cm. in length. Some of the crystals are greatly distorted by flattening parallel to a prism face, and many smaller ones are completely doubly terminated and show no place of attachment to the walls of the pocket. Phenakite crystals ranging up to 1 cm. across were very abundant. The phenakite is fixed in the sequence of mineralization by the fact that the crystals are commonly emplanted on, but not embedded in, smoky quartz. Both single and twin crystals were present, but the single crystals were more abundant. A late-stage introduction of albite followed quartz and phenakite, but the amount of albite introduced, mainly by replacement of microcline, was small. Fluorite was present in good octahedral crystals ranging up to 5 cm. in size. Colorless quartz formed as either small individual crystals or as "caps" on some of the smoky quartz crystals. A tabular summary of the paragenesis of this pegmatite is given in Table 1, column 2. BERYL-PHENAKITE-BERTRANDITE PEGMATITES Two of the pegmatites examined contained all three of the beryllium minerals -- beryl, phenakite and bertrandite. These two pegmatites varied considerably in their paragenesis and will be described separately. Beryl-phenakite-bertrandite pegmatite. This pegmatite is located on the south slope of Mt. Antero. It has been almost entirely excavated by previous collectors, but abundant material on the dump, and information furnished by Over, who collected some fine bertrandite specimens there on previous trips, made it possible to build up a fairly complete picture. The original pegmatite was apparently comparatively large, with occasional small pockets several centimeters across. The pocket minerals of this pegmatite were crystallized on a smaller scale than in most of the other pegmatites seen. The smoky quartz crystals were sharp and well formed but with a maximum length of 3 cm. Beryl formed well-terminated bluish-green crystals up to 3 cm. in length. Crusts of phenakite formed on unetched beryl crystals. Fluorite was present in small colorless and purple octahedra. Bertrandite in both single and twinned crystals up to 1 cm. in length formed as a late-stage alteration of beryl, as shown by its association with deeply etched beryl crystals, or in cavities left by the complete removal of beryl by etching. A tabular summary of the paragenesis of this pegmatite is given in Table l, column 3. Beryl-phenakite-bertrandite fluorite pegmatite. The second pegmatite of this type was found by Montgomery on the northwest face of Mt. Antero. It was completely disintegrated and may not have been in place as seen in the field. It consisted of a cavity about one foot across completely filled with debris, in which the various minerals were buried. None of the material was attached to the walls of the cavity. Microcline was present only in small amount, and was clearly the first formed mineral. It has been deeply etched, with the removal of the perthite lamellae, and later partially replaced by albite. Beryl is not now present in the pegmatite, but its existence at one time is indicated by numerous hexagonal-shaped casts of phenakite. Muscovite in part forms the casts and, therefore, followed beryl in its crystallization. After the removal of the beryl, the phenakite casts were often partially refilled by more phenakite. This later phenakite may have formed directly from the beryl as it was removed by solution. As a general rule, long prismatic twin crystals of phenakite formed later than did the more abundant short prismatic single and twinned crystals. Albite was introduced in small amount toward the close of the period of deposition of phenakite. Fluorite was quite abundant in sharp, well-formed octahedra, and in multiple disk-shaped twins, greatly flattened parallel to an octahedron face. Bertrandite formed in small amount very late in the hydrothermal stage. Quartz is almost entirely lacking. Only two small colorless quartz crystals were found, and their exact place in the sequence of mineralization is not known. A tabular summary of the paragenesis of this pegmatite is given in Table 1, column 4. TOPAZ PEGMATITES Topaz was found in only one pegmatite, on the south side of Mt. Antero. The pegmatite was completely disintegrated and consisted of a mass of fragments in the broken rock. Fragments of graphic granite composed of white microcline and smoky quartz indicated that the walls of the pegmatite were made up of this material. Numerous loose crystals were found of microcline and smoky quartz. There was a small amount of muscovite and fluorite. The topaz was brown in color and consisted of deeply etched crystal fragments. The original crystals must have been several centimeters in length. VEIN DEPOSITS Three veins were examined in the Mt. Antero region. Two of these are in the granite stock and closely associated with pegmatites. The third vein is in the quartz monzonite, about one half mile from the granite contact. Muscovite-quartz vein. This vein was found by Montgomery on the northwest slope of Mt. Antero. It was a narrow, irregular vein with highly altered granite wall-rock on which had crystallized abundant deep green muscovite in clusters of small crystals, and deep smoky quartz in crystals ranging up to 20 cm. in length. Immediately following the smoky quartz, but sharply separated from it, was colorless quartz, which formed either as a capping layer on the smoky quartz or as small druses. Phenakite started to crystallize at this time and in part overlapped with the colorless quartz, as is shown by specimens of phenakite crystals partially or wholly embedded in the colorless quartz which caps the smoky quartz crystals. A small amount of albite was deposited at about this time and is the only feldspar present in the vein. As is shown in Table 1, column 5, phenakite crystallized over a wide range, contemporaneous with quartz, and with colorless and deep purple fluorite. The colorless fluorite built octahedra as large as 10 cm. on an edge. There was then a halt in the crystallization of fluorite, but phenakite continued to form as a surface coating on the colorless fluorite crystals. Purple fluorite was then deposited by building on the colorless fluorite octahedra, and outlasting the period of deposition of phenakite. A pale green coating of fluorite then formed on some of the larger fluorite crystals. Some of the smoky quartz and fluorite crystals are dusted with a drusy coating of purple fluorite, which in this case is cubo-octahedral in habit. Limonite was very abundant, and a small amount of residual pyrite served to demonstrate its origin. When the limonite formed from pyrite it was deposited primarily as a replacement pseudomorph of fluorite. A small amount of native sulphur was also formed. The exact position of the pyrite and limonite in the sequence of mineralization is not known. A tabular summary of the paragenesis of this vein is given in Table l, column 5. Phenakite-quartz fluorite vein. On the northwest slope of Mt. Antero a vertical vein about 4 feet wide is exposed over a length of 20 feet. It is composed dominantly of milky quartz but contains numerous pockets that range in size up to 14 inches across, lined with colorless or light smoky quartz crystals. Phenakite was very abundant, and in part overlapped with quartz in its deposition, as shown by phenakite crystals partially embedded in the quartz that lines the pockets of the vein. Muscovite was present in minor amount, and was followed by orthoclase, variety adularia. Albite was next introduced, principally as a pseudomorph of the adularia crystals. Fluorite was very abundant, sometimes completely filling the pockets. It formed in sharp octahedra up to 10 cm. across. Phenakite is shown to have persisted over a long range in its crystallization by the fact that it is found partially embedded in fluorite. |
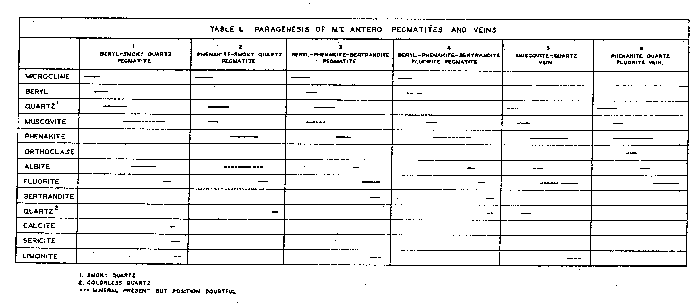
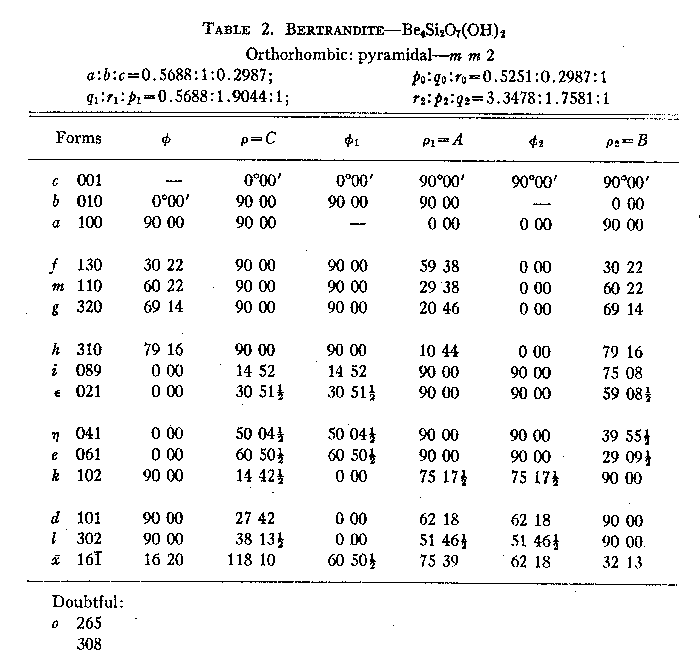
Beryl-quartz-molybdenite vein. This deposit has already been described by Landes (1934). It is only mentioned here to complete the picture of the apparent gradation in the Mt. Antero region from pegmatite to vein. The beryl-quartz-molybdenite vein is not found within the granite stock to which all previously described localities were confined. It is in the surrounding quartz monzonite, about one half mile from the granite contact. The presence of beryl in the vein indicates that the mineralizing solutions arose from the granite stock in which so many beryllium-rich pegmatites are found, and that it can, therefore, be considered as an outlying part of the same general zone of mineralization. SUMMARY AND CONCLUSIONS With the exception of the beryl-quartz-molybdenite vein, the pegmatites and veins of the Mt. Antero region are all found entirely within a single small granite stock, and presumably, therefore, arose from a common source. The paragenetic table (Table 1) of the various deposits described brings out the broad similarity between them, and also several significant differences. These differences are an expression of the physicochemical conditions obtaining during the formation of any particular pegmatite or vein. The most important factors that may have governed the paragenesis of the pegmatites and veins are: (1) chemistry, (2) pressure, (3) temperature and rate of change of temperature, (4) wall-rock control. The paragenetic table shows all of the pegmatites and veins to be similar chemically in that they contain essentially the same elements, as might be expected since they arose from a common source. However, the relative proportions of the elements vary widely in different occurrences. It is probable that the most important influence of variation in chemistry of the primary solutions has been to control the amounts of the various minerals present. Speculation about pressure effect upon the paragenesis of the pegmatites and veins is difficult. All of the bodies are found within a horizon 500 feet thick, and the difference in pressure due to load was not, therefore, very large. More important than this, however, would be the variation in hydrostatic pressure of the solutions, which might be governed by the size of the channel-ways through which the solutions moved. However, since the direct effect of reduced pressure would be to lower the temperature of the solutions, these two factors may be considered to be interdependent. The granite in which the pegmatites and veins are found is very uniform throughout, and the possibility of wall-rock control is slight. The major dissimilarity in paragenesis is between the two groups which have been described as pegmatites and veins. Included in the pegmatite group are all those dikes or lenses of coarsely crystalline microcline and quartz, either together as graphic granite or as individual crystals; that is, those bodies whose early phase at least, was probably magmatic. Included in the vein group are three dikes that are vein-like in shape, and are composed chiefly of typical milk-vein-quartz, with graphic granite and microcline entirely lacking. There can be little doubt that the fundamental differences between pegmatites and veins were caused by temperature of formation. This is brought out clearly in Table l, assuming that from top to bottom in the table is a direction of decreasing temperature. In the true pegmatites (columns 1-4) microcline and beryl were the first minerals to form, and were, therefore, formed at the highest temperature and probably crystallized from a medium close in its properties to a true magma. Following the crystallization of microcline and beryl in the pegmatites came the typical hydrothermal introduction of lower temperature minerals. The veins in their entirety are essentially equivalent to the hydrothermal phase of the pegmatites. The muscovite-quartz vein has highly silicified and altered granite wall rock, and its general nature gives the impression that it was created by the early hydrothermal solutions of a typical pegmatite which escaped from the pegmatite magma chamber into a crack or fissure in the granite. The close association of this vein with several true pegmatites supports this idea. The phenakite-quartz fluorite vein has unaltered, sharp contacts with the granite wall rock and, therefore, appears to have formed at a still lower temperature than the muscovite-quartz vein. For the same reason the beryl-quartz-molybdenite vein apparently formed at a relatively low temperature. Apparently all the pegmatites started to crystallize at about the same temperature. The paragenesis of the individual pegmatites is broadly similar. The lack of certain minerals in some which are present in others, and the differing amounts of the various minerals, can be attributed to slight compositional differences in the solutions which introduced the hydrothermal minerals, and to a varying rate of change of temperature. The paragenetic table (Table 1) shows phenakite to be stable at a lower temperature than beryl, and quick cooling past the stability range for beryl might prevent its crystallization, resulting in the formation of phenakite at a lower temperature. Temperature of formation. Any precise statement as to the temperature of formation of a pegmatite is of doubtful value. However, for at least some of the pegmatites of the Mt. Antero region (the beryl-smoky quartz type), it is known that the smoky quartz formed as low-quartz, and thus crystallized at some temperature below 573°C.1 Since smoky quartz was the third mineral to start to crystallize it seems probable that the upper temperature limit of the pegmatites could not have been much greater than 600°C. It has been established by various investigators that the temperature of formation of adularia is about 230°C. On this basis the lower temperature of formation of the phenakite-quartz fluorite vein will be some figure below this value, perhaps as low as 100°C. DESCRIPTION OF THE MINERALS The common pegmatite minerals microcline, muscovite, quartz and albite of the pegmatites of the Mt. Antero region have no features requiring a detailed description. A description will be given, however, of the less common minerals bertrandite, beryl, fluorite and phenakite. BERTRANDITE Bertrandite was first described from Mt. Antero by Penfield in 1889. The material collected by the writer can add nothing to Penfield's description. However, a structural study of bertrandite by Ito and West (1932) has shown that the unit chosen by Penfield does not correspond to the true unit as revealed by x-ray study. The change necessary for correspondence is a minor one, and consists only of halving the c-axis of the Penfield setting. The transformation is given by 100/010/00½. The unit cell dimensions of Ito and West are ao=15.19Å, bo=8.67Å, co =4.53Å. From this the axial ratio ao:bo:co=0.571:1:0.298 is obtained. (To obtain this conventional form for the axial ratio the ao and bo lengths of Ito and West must be interchanged.) This is in good agreement with the axial ratio obtained by halving c of Penfield's original values. A new angle table has been calculated for all known forms on bertrandite, using the elements required by the structural study of Ito and West. It is evident that the change made in morphology requires no revision of previous statements of physical and optical properties. |
Bertrandite from Mt. Antero is found in platy crystals tabular parallel to c{001} and usually elongated parallel to the a-axis. The crystals are white or colorless and transparent, and range in size up to 1 cm. in length. Their hemisymmetric character is indicated by the fact that one basal plane is commonly flat, while the other is rounded and striated parallel to the a-axis by the oscillatory combination of the basal plane with a dome, probably ε {021}. Twin crystals are relatively common. The twin plane is (021). The twins are heart-shaped in cross section, with the smooth basal plane on the outside. The appearance of single and twinned crystals of bertrandite from Mt. Antero is illustrated by Figs. 3 and 4. |
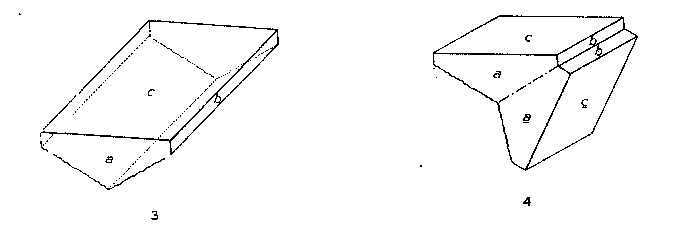
FIG. 3. Bertrandite from Mt. Antero, Colorado. FIG. 4. Bertrandite, twin crystal. Twin plane (021). Mt. Antero, Colorado. BERYL Beryl, variety aquamarine, is the principal mineral for which Mt. Antero has long been known. Only the beryl which has grown freely in open pockets of the pegmatites is transparent and flawless, and, therefore, of value as gem aquamarine. Unfortunately much of the beryl is found frozen into the pegmatite and is then always translucent and worthless. The color of the beryl from the Mt. Antero region varies from pale greenish blue to fairly deep aquamarine. The largest crystals ever found in this region were found by Over in 1932, on White Mountain. The contents of this pocket are now in the Mineralogical Museum of Harvard University. The largest crystal is 3.5X20 cm., gemmy in part. Several others almost as large were also taken from this pocket, and many more of smaller size. Some pegmatites contain beryl crystals that are totally unetched. In others the degree of etching may vary to the extreme case in which the crystals have been reduced to a mass of fibres of beryl, or have been completely redissolved. Commonly the beryl crystals are terminated only by a basal plane. The terminal forms p {1011} and s {1111} were also observed on numerous crystals. Sometimes the prism zone is sharp and other times deeply striated parallel to the length of the crystals. Figure 5 illustrates part of the beryl crystals found in the beryl-smoky quartz pegmatite. |

|
FIG. 5. Beryl from White Mountain, Colorado. FLUORITE The fluorite of the pegmatites and veins of the Mt. Antero region is always octahedral in habit. Octahedra of fluorite were found ranging in size from 1 mm. to 20 cm. Deep purple is the most common color but small colorless crystals were also found, and occasionally light green fluorite formed as a thin coating on earlier formed crystals. Fluorite crystals were found in the beryl-phenakite-bertrandite pegmatite which are twinned on an octahedron face, and show dominantly the octahedron form, with occasional small truncating faces of the dodecahedron. About 15 twin crystals were found in this pegmatite. Most of these are much flattened parallel to an octahedron face, and with one individual of the twin developed to a much greater degree than the other, as shown in Fig. 6. Others are disk-shaped and made up of several individuals.
Two fluorite twins were found in the muscovite-quartz vein. These twins are typical "spinel" twins, made up of two equally developed octahedra, twinned on an octahedron face. The best formed of these two twins is about 5 cm. across and deep purple in color, with a surface coating of pale green color. Also found in the muscovite-quartz vein were drusy coatings of minute purple fluorite crystals upon smoky quartz and fluorite. These drusy coatings are cubo-octahedral in habit, with the cube and octahedron faces nearly equally developed. Since the drusy crystals were clearly the last formed fluorite of the vein, and, therefore, formed at the lowest temperature, it seems probable that temperature was the dominant factor in controlling the habit of the fluorite, and that octahedral habit is favored by high temperature. What the temperature of crystallization of the cubo-octahedral crystals was is not known. However, the association of octahedral fluorite with adularia in the phenakite-quartz-fluorite vein indicates that octahedral fluorite can develop at a temperature somewhat less than the temperature of crystallization of adularia. Temperature as a controlling factor of the habit of fluorite has also been suggested by Drugman (1932). PHENAKITE Introduction. In order to properly describe the phenakite from Mt. Antero it was found necessary to make a general survey of the whole phenakite problem, principally in order to determine whether the present morphology and crystal structure were in agreement. The unit cell and structure of phenakite was determined by Bragg in 1927, and the unit cell dimensions by Gottfried at about the same time (1927). Later (1930) Bragg and Zachariasen further elaborated on the phenakite structure. Pough (1935, 1936) made a detailed study of the morphology of phenakite but failed to point out the direct tie-up between morphology and structure. Symmetry. Following the method outlined by Buerger and Parrish (1937), a series of equi-inclination Weissenberg photographs about the c-axis permits one to resolve the reciprocal lattice into stacks of plane reciprocal lattices normal to the c-axis. The projections of these lattices are displaced over each other by 1/3 of the long mesh diagonal of each layer, thus fixing the space lattice type as rhombohedral.
The symmetry class is determined by the relation of the third-order terminal
planes on both top and bottom of the crystals. Such planes are only repeated by
a three-fold axis and an inversion center. The Weissenberg photographs also
clearly reveal the absence of any vertical symmetry planes. The crystal class
is, therefore, C3i (R3). Of the two space groups based on this crystal class
only one is based on a rhombohedral lattice; thus the space group is C3i2
(R Unit Cell. The unit cell chosen by Bragg is a rhombohedron with the length of the rhombohedron edge 7.684 Å and a=108°01'. In order to bring out the relation between structure and morphology a structural cell based upon a hexagonal network must be used. The rhombohedral unit cell dimensions of Bragg transformed to the corresponding hexagonal cell are shown in Table 3. Also listed are the unit cell dimensions determined by the writer.
TABLE 3
The hexagonal unit cell of the writer has V0= 1097 cubic Å and contains 18 (Be2SiO4). The calculated density is 2.98 (measured 2.97-3.00). From the unit cell dimensions a calculation of the axial ratio yields ao:co=1:0.6645, and the polar elements po:ro=0.7673:1, which is in good agreement with the morphological elements of Goldschmidt and Schröder (1909). Morphological elements. The morphological presentation of a rhombohedral mineral can be greatly simplified by omitting a treatment of' Goldschmidt's alternative G1 and G2 positions, as has been pointed out by Peacock and Bandy (1938). This has been done in the new morphological elements for phenakite given below. The elements of Goldschmidt and Schröder (1909) have been used, but recalculated to the proper position. Since the φ and ρ angles remain as given by Pough (1936) a complete new angle table is not required.
TABLE 4
It is interesting to note that seven forms of the list of certain forms given
by Pough do not fit the rhombohedral rule, which states that h+i+l=3n. These are
c{0001}, m{10 Phenakite from Mt. Antero. Phenakite is found at Mt. Antero in a. variety of habits and sizes. The crystals are opaque white to colorless, sometimes transparent and with a faint yellow tinge. Both single and twin crystals are abundant. In size they range from 3½ cm. to ½ mm.
The forms measured on Mt. Antero crystals by the writer, listed in order of
importance, are: prisms a{11
By far the most common habit of phenakite from Mt. Antero is short prismatic
single crystals, as shown in Figs. 7 and 8. It is characteristic of practically
all Mt. Antero phenakite to be terminated dominantly by ; X·{ |
Although no exact rule can be formulated, in general it appears that low
temperature of crystallization favors development of twin crystals of phenakite,
and also favors the most extreme development of prismatic habit of the twins.
REFERENCES
BRAGG, W. L., The structure of phenacite: Proc. Royal Soc., A, 113, 642-657
(1927). BRAGG, W. L., and ZACHARIASEN, W. H., The crystalline structure of
phenacite and willemite: Zeits. Krist., 72, 518-528 (1930).
BUERGER, M. J., and PARRISH, W., The unit cell and space group of tourmaline:
Am. Mineral., 22, 1139-1150 (1937).
CRAWFORD, R. D., A contribution to the igneous geology of central Colorado: Am.
J. Sci., (5), 7, 365-388 (1924).
CROSS, R. G., Notes on aquamarine from Mt. Antero: Am. J. Sci., (3),33,161-162
(1887). DRUGMAN, J., Different habits of fluorite crystals:
Mineral. Mag., 23,
137-144 (1932). GOLDSCHMIDT, V., and
SCHRÖDER, R., Phenakit aus Brasilien: Zeits. Krist., 46, 465-470 (1909).
GOTTFRIED, C., Ober die Struktur der Phenakitdioptasgruppe: Neues Jahrb. Min.,
Beil. Bd. 55, A, 393-400 (1927).
ITO, T., and WEST, J., The structure of bertrandite: Zeits. Krist., 83, 384-393
(1932). LANDES, K. K., The paragenesis of the granite pegmatites of central
Maine: Am. Mineral., 10, 355-411 (1925).
___________ The beryl-molybdenite deposit of Chaffee County, Colorado: Econ.
Geol., 29, 697-702(1934).
MONTGOMERY, A., Storm over Antero: Rocks and Minerals, 13, 355-369 (1938).
PEACOCK, M. A., and BANDY, M. C., Ungemachite and clino-ungemachite: New
minerals from Chile: Am. Mineral., 23, 314-328 (1938).
PENFIELD, S. L., Phenacite from Colorado: Am. J. Sci., (3), 33, 131-134 (1887).
___________
Bertrandite from Mt. Antero: Am. J. Sci. (3), 36, 52-55 (1888).
_________ Some observations on the beryllium minerals from Mt. Antero: Am. J.
Sci., (3),
40, 488-491 (1890).
PENFIELD, S. L., and SPERRY, E. S., Mineralogical notes: Am. J. Sci., (3), 36,
32 (1888). POUGH, F. H., The morphology of phenacite from two new occurrences:
Am. Mineral., 20, 863-874 (1935),
_________, Phenakit, seine Morphologie and Paragenesis: Neues Jahrb. Min.,
Beil. Bd.
71,A, 291-341 (1936).
OVER, E., JR., Mineral localities of Colorado: Rocky and Minerals, 3, 110-111
(1928). __________ Further explorations on Mt.
Antero, Colorado: Rocks and Minerals, 10,
27-29 (1935).
SMITH, W. B., Mineralogical notes, 1, 2, and 3: Colorado Sci. Soc., Proc., 2,
177-179 (1887). NOTE 1 The exact value of this inversion is, of course, unknown, since the pressure
at the time of formation is not known.
[var:'startyear'='1939'] [Include:'footer.htm']
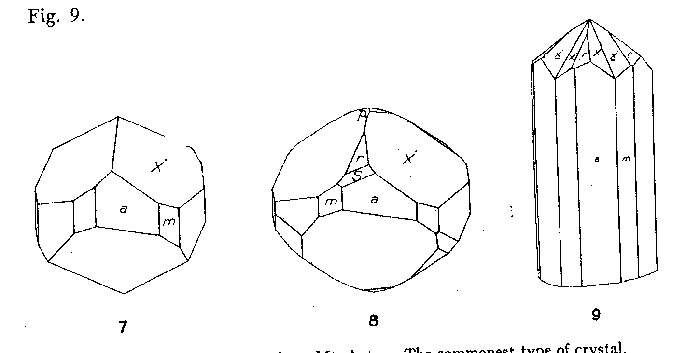
FIG. 7. Crystal of phenakite from Mt. Antero. The commonest type of crystal.
FIG. 8. Crystal of phenakite from Mt. Antero. FIG. 9. Interpenetration twin of
phenakite from Mt. Antero. Twin plane (0001).