|
|
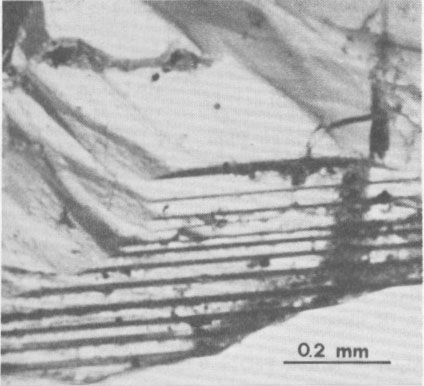
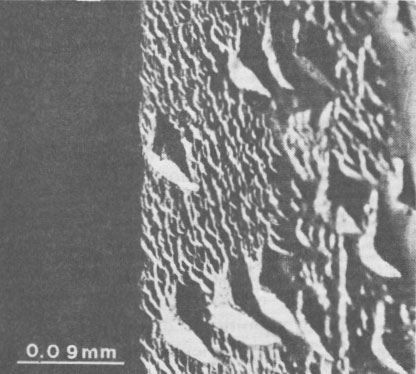
Volume 65, pages 183-187, 1980 Euclase from Santa do Encoberto, Minas Gerais, Brazil GIORGIO GRAZIANI AND GIOLJ GUIDI Istituto di Mineralogia e Petrografia, Università degli Studi Abstract Transparent euclase from Santa do Encoberto, Minas Gerais, Brazil, was studied with regard to optical properties, hardness, specific gravity, IR spectra, and X-ray data. Thermal analyses, TG, DTA, and DSC were also conducted. Microprobe analyses showed: SiO 2 = 41.61%, A12O3 = 34.76%, FeO = 0.28%, BeO = 16.95%, and H2O = 5.95%.The euclase crystals possess growth surface terraces and striations oriented parallel to (hkl)-(hk0) and (hkl)-(100) edges and natural triangular etch pits on the faces of the prism (670). Minute inclusions consist of various clusters, not previously described for this mineral, of hexagonal crystals of apatite, corroded and irregular plates of hematite, minute needles of rutile, and rounded grains of zircon. Introduction The occurrence of euclase in the famous mineralogical area of Ouro Preto, State of Minas Gerais, Brazil was once thought to be limited to a few localities, but in the past few years other discoveries in the northeast of this state have acquired notable mineralogical renown. In particular, doubly terminated transparent or milky euclase crystals up to severed centimeters in length have been reported from Santa do Encoberto, county of São Sebastião do Maranhão. The deposit consists of a heterogeneous beryl-bearing pegmatite vein, enriched with black tourmaline, and enclosed in mica schists. Euclase is normally found in druses with mica and albite, and in small lenses near the quartz core. The paragenesis includes mica, albite with microcline, vermiculite, quartz, calcite, apatite, pyrrhotite, pyrite, spessartine, and secondary uraninite and limonite with altered feldspar near the surface (Cassedanne and Cassedanne, 1974). No complete study had been made of the euclase from this locality. Recent optical research by Bank (1973) determined that the birefringence was high but not the highest known (Sharp, 1961). It seemed appropriate to conduct the present study to determine: (1) the mineralogical characteristics of the euclase from Santa do Encoberto, (2) the nature of inclusions in the euclase, and (3) possible genetic crystallization conditions of the embedding euclase. Material and methods The fragmented specimens (ca. 4 mm x 3 mm) of slender, transparent euclase crystals from Santa do Encoberto were kindly made available by Professor W. F. Eppler, Munich, West Germany. Professor Eppler brought to our attention the presence of thin, irregularly formed, black inclusions composed of groups of crystals, with metallic luster and occasioned frayed edges. The (hkl) faces were in contact with the pinacoid {100} and the prism {hk0} and were characterized by oriented surface terraces which exhibited successive accretion (Fig. 1). The steps of growth were evidenced by tiny black dots which accentuated the borderline. Optical observations and geometric considerations revealed that the striations were oriented parallel both to the (hkl)-(hk0) and the (hkl)-(100) edges. Cleavage was perfect along the pinacoid {010} and imperfect along the prism (110). Careful optical observations of the prism faces (670) revealed a multitude of pits due to natural etching (Fig. 2). These oriented pits were triangular and composed of three faces asymmetrically arranged, sometimes curved. These triangular pits were often distorted; the longest side was prolonged in a beak-shaped appendix and indicated the direction of greater solubility.
Isolated pits were identified based on variable densities within crystal faces, over which there were micropits displaying a random distribution. Size and depth measurements were made of isolated pits. An average ratio of about 1.48 was found between the longest (ca. 80 µm) and shortest (ca. 54 µm) sides of the triangle, whose depth was about 10 µm. The presence of natural etch pits on the rough faces of the prism (670) of Brazilian euclase has been pointed out in a careful crystallographic study (Piazza, 1926) of six magnificent crystals in the Museum of the Istituto di Mineralogia e Petrografia of the University of Rome. The museum specimens (sample Nos. 13923/1, 13924/2, 13925/3, 13926/4, 19210/8, 23005/9) were credited to Ouro Preto, state of Minas Gerais, Brazil. The shape and orientation of the cavities on the rough faces of these crystals were similar to those of the Santa do Encoberto euclase (Fig. 2). Optical observations were made on thin sections with different orientations, in white plane-polarized light. A universal stage was used to measure the optical angle and to determine refractive indices directly by the immersion method. A water cell, connected with the central plate, controlled the circulation and the liquid temperature within the range of ca. 50°C. An Abbe refractometer connected in parallel to the water system enabled the determination of the refractive index of the mounting liquid (Cargille Laboratories) at any time. The absorption spectrum was observed by a Gübelin spectroscope. Several Vickers hardness measurements (10 determinations) were made with a Leitz hardness-indentor on the polished faces of the pinacoid {010) with 200 g load ranging from 1000 to 2000 kg/mm 2. The density was measured on a Berman balance and was calculated from unit-cell parameters.Infrared spectra were examined in the region 3700-4000 cm -1 on a Perkin Elmer 577 filter-grating spectrometer, using 0.5 mg of euclase in 12 mm KBr disks. The sample was prepared as described by Russell (1974).Differential thermal analyses were conducted on a BDL micro-differential thermal analyzer equipped with a semi-micro probe (capacity: 25 µl). The thermocouples were made with Platinel II and the experiments were conducted in a dry nitrogen current, 5 cc/min, with a thermal gradient of 10°C/min, and in the 25-1300°C temperature range. Under the same experimental conditions, thermogravimetric analyses were carried out on a Cahn RG electro-thermal balance and the calorimetric determinations on a differential scanning calorimeter Mettler TA 2000L. About 10 mg of material was used for each experiment. X-ray powder diffractograms were obtained with Ni-filtered CuKα radiation with five oscillatory scans at 1/4° 2θ per minute from 10° to 50° 2θ. Unit-cell parameters were estimated from least-squares refinement of X-ray powder diffraction data (Farinato and Loreto, 1975) using semiconductor-grade silicon (Janel Ash JM, spec. impurity < 300 ppm) as an internal standard. Experiments were conducted under the same experimental conditions using an X-ray powder diffractometer Philips PW 1420 with a heating camera, type AF 3000. The power was supplied by a high-frequency generator (3 kW), and the automatic temperature controller unit had a 30 to 1500°C range. The diffraction angles were calibrated by using, for all temperatures, pure α-A12O3 for chromatography heated at 1500°C (Merck's reagent, spec. impurity < 100 ppm) as internal standard.
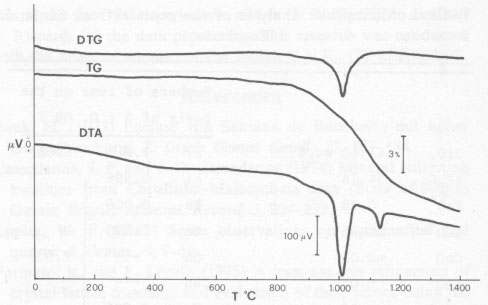
Electron microprobe analyses were carried out on a Jeol JSM-50A using a series of natural and synthetic standards. Matrix corrections were made according to the program EMPADR VII described by Rucklidge and Gasparrini (1969). All iron was calculated as FeO. Sample metallization with graphite did not enable the detection of beryllium, which was determined with pyrophosphate (Vogel, 1971, p. 518-519). Results Optical properties were determined on thin section displaying an acute bisectrix figure with a 2 V of about 60° (optically positive). The refractive indices were: α = 1.651, β = 1.657, γ = 1.675, γ - α = 0.024. The absorption spectrum featured bands at 4680Å in the blue region, and a doublet in the red region at 7050Å characteristic of euclase. The density measured was 3.065±0.005 g/cm 3 and that calculated 3.110±0.003 g/cm3. The average microhardness was 1310 kg/mm2; this value corresponds to a Mohs hardness of ca. 7½. The infrared spectrum was examined under the experimental conditions used by Yegorov (1967). The spectrum showed a marked splitting of the band between the measured wavelengths from 400 to 1300, and from 3200 to 3700 cm-1.According to Yegorov the absorption bands evident in the interval 500-830 cm-1 are characteristic of BeO, tetrahedral oscillations in beryllium minerals. Bands in the interval 830-1060 cm-1 are due to the oscillations of SiO 4 tetrahedra. Moreover, the 1070 cm-1 band is probably due to the overlapping of the SiO4 and BeO, tetrahedral bands, whereas the intense and single band around 3585 cm-1 marks the valence oscillations of the OH groups, whose presence was proved by the heating experiments.The unit-cell parameters, determined on an annealed sample, were: a = 4.771±0.003, b = 14.308±0.010, c = 4.631±0.004Å, ß = 100.33±0.07°; V = 311±1A 3; Z = 4.The thermogravimetric curve (Fig. 3) showed that at ca. 800°C a loss of weight began which culminates at ca. 1012°C. The clear endothermal peaks on DTG and DTA (Fig. 3) showed the same effect. At ca. 1130°C, the thermodifferential curve was characterized by a small but well-defined endothermal peak with no corresponding weight loss.
Calorimetric measurements were made in the weight loss range (800-1050°C) and established that the transformation heat, resulting from the loss of the OH group, was equal to ca. 115 cal/g. Thermal analyses allowed the identification of three fields of stability: the first ca. 25-800°C, the second ca. 1050-1100°C, and the third over ca. 1200°C. Heating X-ray powder diffractometer experiments were conducted in order to identify the phases formed in the temperature ranges previously defined. X-ray powder diffractograms showed that the euclase was stable up to ca. 800°C. Between 1050°C and 1100°C the formation of a phase corresponding with the data for beryllium aluminum silicate 2BeO·11/3Al2O3·6SiO2 (Card 18-204 of the JCPDS) was observed. Over 1100°C, the structure of beryllium aluminum silicate began to break down. In order to ascertain possible variations of the optical and crystallographic properties of the euclase, heating experiments were conducted over the range of proven stability of the mineral (25°-800°C). At 200°C intervals, the sample was placed in an electric furnace for 20 minutes (using a dry nitrogen current of 5 cc/min) and then cooled. The optic axial angle 2V, the diffraction indices, and the unit-cell parameters were then determined for each cooled sample. Optical and crystallographic parameters remained almost constant in this temperature range. In order to investigate the chemical composition of the euclase more thoroughly, samples were analyzed by an electron microprobe. The results are reported in Table l. Table 1. Microprobe analysis of the euclase from Santa do Encoberto
1 total iron as
FeO.
Traces of Pb, Ge, and F were found and the samples were checked for Ti, Mg, Ca, and Mn. The ignition loss, determined thermogravimetrically on a fragment of pure euclase, occurred at 850°C. By using a mass spectrometer, it was possible to ascertain that only water vapor was emitted during heating at 850°C. Therefore, the weight loss was attributed totally to H2O+. These results are in agreement with an ideal formula for euclase, AlBeSiO4(OH).
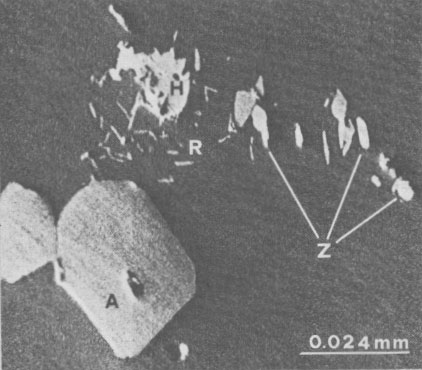
It seemed appropriate, by using an electron microprobe, to study the tiny black inclusions which, because of their small size (ca. 0.010 mm X 0.005 mm), could not be identified by optical observations. Figure 4 shows that the inclusions consist of an accumulation of different minerals distributed in clusters. The presence of P2O5 (42.20%) and CaO (55.78%) indicates that the large hexagonal crystals (ca. 0.036 mm X 0.026 mm) are apatite. Since chlorine and fluorine were not detected, the hexagonal phase is probably carbonate or hydroxyapatite. Small corroded and irregular plates (ca. 0.016 mm x 0.016 mm) can be seen near the center of Figure 4. The high Fe2O3 content (98.92%) and small quantity of TiO2 (0.010%) suggest that these plates are hematite. Sprinkled among the small plates of hematite and the nearly hexagonal apatite are slender crystals of acicular appearance (ca. 0.005 mm X 0.001 mm). These minute needles essentially consisted of TiO2 (98.82%) with traces of FeO (0.50%); they were therefore identified as rutile. Rounded grains (ca. 0.010 mm X 0.002 mm) consisting of ZrO2 (67.12%) and SiO2 (32.50%) occurred among the rutile needles. These were therefore identified as zircon, although their structure was not established. Discussion and conclusions The study of this uncommon euclase from Santa do Encoberto enables us to advance some hypotheses regarding its formation. The presence of isolated triangular etch pits, whose disposition agrees with the monoclinic symmetry of euclase, is probably due to corrosive alkaline solutions. A mature stage of the etch is confirmed by the pit distortions. The notable depth of the isolated pits and their distribution in groups on the faces of the prism (670} suggest that the pits nucleated at dislocations. Growth steps on the (hkl) faces may explain the isolated pits, while micropits which completely cover the faces may be attributed to nucleation upon defects such as interstitial vacancies (Patel and Goswami, 1962). The orientation of the faces delimiting each etch pit suggests that they are cavities bounded by three planes, ascribable to high index faces which show a low reticular density. The faces of the prism (670), too, feature a lower density due to a greater instability with respect to other (hk0) faces. The tiny black inclusions representing the boundaries of the growth steps in the euclase crystal are suggested to be nuclei for further growth of the embedding mineral (Vacher, 1977). These frayed inclusions of metallic luster, preferentially aligned along these planes, consist of clusters of crystals with refractive indices higher than those of the embedding euclase (Figs. 1 and 4). A very similar disposition of inclusions in a ghost quartz, embedded in rock-crystal from Brazil, is described by Eppler (1963). The inclusions consist of a multitude of very minute, unidentified black crystals and chips of quartz which emphasize the boundary between ghost quartz and rock-crystal. The presence of hematite plates surrounded by high refractive index minerals explains the metallic and frayed appearance of the inclusions. In particular, the rounded zircon grains and the irregular hematite plates were presumably formed before the euclase, whereas the subhedral apatite crystals and the rutile needles were probably formed concurrently. The minerals associated with the euclase of Santa do Encoberto, described by Bank (1973) and Cassedanne and Cassedanne (1974) cannot be referred to a single paragenesis. In particular, microcline, albite, spessartine, and quartz associations in large crystals suggest high temperature and moderate pressure, whereas the presence of vermiculite denotes low temperature. Acknowledgments We thank Professor W. F. Eppler, Munich, West Germany, for his assistance and comments on this study. Thanks are also extended to Dr. H. Wiedeman, Mettler Instrument of Zurich, Switzerland, for the DSC experiments, and to Dr. V. Di Giulio and Mr. S. Riccardi for the data processing. This research was conducted with the financial support of the Italian C.N.R., CT 77.00802.05. References Bank, H. (1973) Euclase von Santana de Encoberto mit hoher Doppelbrechung. Z. Dtsch. Gemm. Gesell., 22, 183-184. Cassedanne, J. P. and J. O. Cassedanne (1974) Mineral collecting localities from Capelinha-Malacacheta area (State of Minas Gerais, Brazil). Mineral. Record, 5, 224-232. Eppler, W. F. (1963) Some observations on aquamarine and quartz. J. Gemm., 9, 9-16. Farinato, R. and L. Loreto (1975) A least-squares refinement of crystal lattice constants and evaluation of their errors, using the direct unit-cell. Rend. Soc. Ital. Mineral. Petrol., 31, 486-500. Patel, A. R. and K. N. Goswami (1962) A new method for the study of dislocation etch pits. Z. Kristallogr., 117, 81-91. Piazza, M. (1926) Studio cristallografico di alcuni cristalli di euclase del Brasile. Mem. Accad. Naz. Lincei, S. VI, Rend. CL Sc. Fis. Mat. Nat., 2, 17-29. Rucklidge, J. and E. L. Gasparrini (1969) Electron Microprobe Analytical Data Reduction. EMPADR VII. Manual reissue, Department of Geology, University of Toronto, Toronto, Canada. Russell, J. D. (1974) Instrumentation and techniques. In V. C. Farmer, Ed., The Infrared Spectra of Minerals, p. 11-25. Mineralogical Society, London. Sharp, W. N. (1961) Euclase in greisen pipes and associated deposits, Park County, Colorado. Am. Mineral., 46, 1505-1508. Vacher, A. (1977) Etude sommaire des cavités tubulaires dans des aigues-marines. Rev. Gemm. Assoc. fr. Gemmol., 50, 4-6. Vogel, A. I. (1971) A Text Book of Quantitative Inorganic Analysis including Elementary Instrumental Analysis. Longmans, London. Yegorov, I. N. (1967) Euclase from quartz-chlorite zones of altered Yakutian granite. (in Russian) Dokl. Akad. Nauk. SSSR, 172,433-436.
Manuscript received, December 4, 1978; [var:'startyear'='1980'] [Include:'footer.htm'] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||