|
|
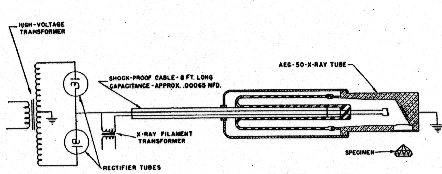
Volume 32, pages 31-43, 1947 FREDERICK H. POUGH, The American Museum of Natural History, New York City, AND T. H. ROGERS, Machlett Laboratories, Inc., Springdale, Connecticut. ABSTRACT Irradiation of gemstones with a new and powerful beryllium windowed x-ray tube effects, in a few minutes, color changes which with previous tubes either gave negative results or required hours of exposure. Some of the effects appear to be permanent, some disappear slowly upon exposure to light, and some revert without other stimulation. The anticipated availability of this treatment to temporarily improve commercial gems makes it important that jewelers be informed of the possibility so that they may be on their guard against frauds of this nature. The recent development of a new and extremely intense source of x-rays (Rogers, 1945-1946) by the Machlett Laboratories suggested the possibility that irradiation experiments with this equipment might materially shorten the time required for producing coloration effects, long studied by physicists, in various minerals and reveal colors which had not previously been affected by x-rays produced by ordinary tubes. In the early days of experimentation with various energy sources, it was noted that both radium and x-rays affected the coloration of various minerals. Doelter, 1912, lists a number of such changes: in diamond, spodumene, quartz and topaz, among others. The mineralogists of that day had no explanation for this, or for some of the other phenomena, like fluorescence, all of which were recorded on a basis of observation. On the other hand, in more recent years, physicists working with transparent mineral materials have observed various color changes as incidental phenomena in the course of other experiments, such as photoconductivity. For purposes of comprehensive explanation and the working out of theories, however, they have concentrated in a few fields of simple compounds, especially the alkali halides.1 The more complex phenomena involved in crystals with a large number of constituents still require explanation, although it is natural to suppose that similar mechanisms will operate in these cases. The nature of the changes induced by x-ray radiation, however, as they are explained at present by the physicists, makes it unlikely that any permanent results will be obtained from this type of attack. The slight disruption of the electronic structure depends upon the existence of structural defects, into which electrons freed from halogen ions can migrate and become trapped when released by the x-ray bombardment. However, since this irradiation results in an unstable structure within the affected crystals with unsatisfied positive and negative charges, comparatively little energy is required to move the electrons back into a stable combination with the consequent elimination of the electrons trapped at the lattice defects, and a return to the normal state. Some substances, those that fluoresce and phosphoresce, for example, are in an easily excited state when they crystallize. Others are more stable, and while they may be colored by the irradiation, they show little or no luminescence. A third group appears to be entirely unaffected by the bombardment; though no experiments have ever been performed with most of them to see whether or not they became conductors during such an irradiation, and consequently had been affected, even though it had not subsequently shown up as a color change. The irradiation of gem stones, and the possibility that some day a method may be found for making permanent the temporary effects now observed is, of course, an intriguing problem and one which naturally immediately presents itself when a new powerful source of x-rays becomes available. The radiation output of the Machlett beryllium window tube is equivalent to several hundred times the output of tubes previously available. This means that effects can be obtained in one minute that would require several hours with a conventional x-ray apparatus. The stones selected for the study were dictated by previous experience with especially sensitive material, such as gem spodumene; by commercially desirable changes, if they could be made permanent, such as those found to occur in the corundum gems; and by various other interests. The results of the exposures will be found in the table. Special phenomena observed in the several stones will be discussed under those headings. The specimens listed in Table 1 were all subjected to radiation from a Machlett Type AEG-50 x-ray tube (Fig. 1) having a focal spot approximately 5 mm. wide. The tube was energized by a full-wave rectified non-filtered power supply, in accordance with the circuit diagram of Fig. 2. The voltage applied to the tube was 50 kilovolts peak, as measured by the spark-over distance between standard 12.5 cm. spheres. The current through the tube was adjusted to 50 milliamperes as measured by a d.c. milliammeter.
The Type AEG-50 tube is provided with a beryllium window approximately 1 mm. in thickness, located 2 cm. from the center of the focal spot. The target is of tungsten, positioned at an angle of 20° to the central axis of the x-ray beam, which covers a conical solid angle of 40°. The specimens were placed approximately 0.5 cm. from the window.
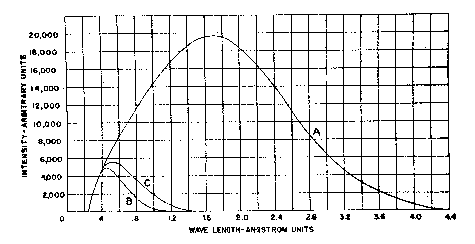
The x-ray intensity at this point was not measured directly, its order of magnitude being estimated with a sufficient degree of accuracy from data given by Rogers (1945-1946) for measurements made on a similar tube of 50 MA and 50 KV constant potential, under which conditions a value of 2,330,000 r per minute was obtained at the window. A correction for the fact that in the present case the voltage is pulsating rather than constant potential, plus the correction for the additional distance from the focal spot, is estimated to be approximately one-half, indicating a dosage at the surface of the specimens in the order of one million r per minute. The quality of these rays (see Fig. 3) is such that they are very readily absorbed, even by low atomic number materials such as comprise most of the mineral specimens studied. The color changes observed in the larger specimens indicated a penetration of the radiation to a depth of approximately 1 cm. in sufficient intensity to produce perceptible reaction.
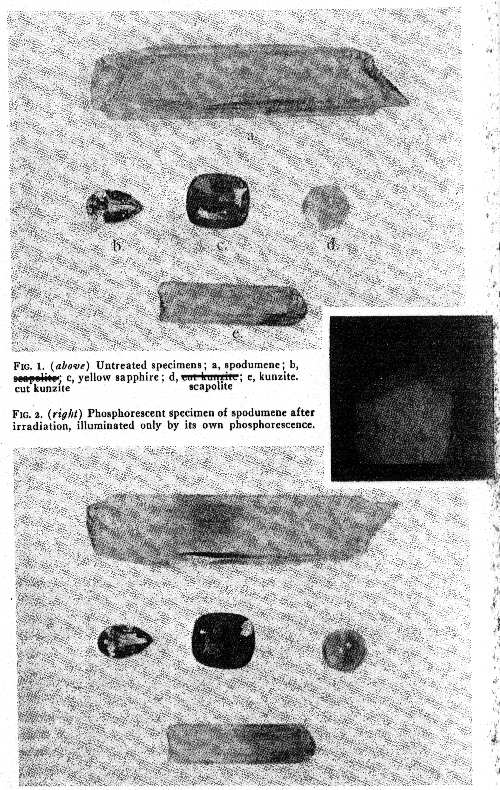
DISCUSSION OF MINERALS TESTED Spodumene Various specimens derived from several sources were treated and comparable results were obtained, no matter what the source. The light green coloration is very intense but soon fades when exposed to sunlight or on heating, and the stone reverts to its original appearance. Treated stones kept in the dark appear to retain their color indefinitely. The coloration is rapid, but appears for a time to progress through the crystal at a definite rate, a five minute exposure, for example, produces a colored layer a little more than 1 mm. thick. The most remarkable feature in the change in spodumene (the kunzite type was the variety treated most frequently), was the customary intense and persistent orange phosphorescence, which made it look like a glowing coal when it was removed from the tube and which could be seen for as much as an hour. The appearance of a green coloration was directly related to this phosphorescence, for immediately after the cessation of irradiation, the stone observed had a brownish hue in the light, and the green did not become visible until the phosphorescence had ended. Heating renewed the phosphorescence, and removed the green coloration. One specimen, a white Brazilian crystal, showed in one area no phosphorescence but the irradiated area was green when it was removed from the window of the tube. The portion which phosphoresced was sharply separated from the non-phosphorescent part immediately following the exposure, but no such demarcation was observable after the phosphorescence had ceased and the stone assumed a uniform green. It is surprising to note that Doelter obtained no color change in this mineral by x-ray irradiation. Beryl A series of differently colored stones were treated and found to be affected but slowly by the irradiation. The first, a pale aquamarine, showed no change after eight hours of exposure. This stone was given prolonged treatment, a total of forty-one hours, and at the end of that time the stone was a light green color, and was a very attractive gem. All of the very pale blue stones became light green after treatment, but this color change was attained only after long exposures and was relatively light. A deep blue aquamarine became moderately green after prolonged (50 hrs.) treatment. In fading experiments the color became somewhat lighter and yellower, but could not be entirely removed. The stone has not been heated and remains green. An emerald given a short exposure was not affected and a pale colorless beryl became light brown after sixteen hours of exposure. There was no fluorescence or phosphorescence accompanying or following these exposures, though the calcite on a Colombian emerald matrix specimen phosphoresced. On heating, the irradiated stones reverted to their original color, without heat they appear to have been permanently colored. Corundum The most unexpected results were those obtained with various corundum gems, most marked in the case of the pale natural Ceylon sapphires. Previous reports had not indicated that sapphire would be particularly susceptible to changes induced by irradiation, but it was found that sapphires were, with spodumene, the most easily altered of all the gem stones tested, and that the effects disappeared on exposure to light more slowly than might have been expected from the rapidity of the coloration. The color added by the x-rays was the same in each case, but variations in the original color naturally affected the result. The actual induced color is a rich golden or amber hue, in the case of colorless or pale sapphires this would be a most desirable change if it could be made permanent. Completely colorless sapphires become a rich amber in an exposure of five or ten minutes; usually the beam was directed at the culet so that the effect on the facetted gem would be most pronounced. Viewed horizontally, these stones are seen to be deeply colored near the surface where the radiation first struck the gem, but fade out to colorless a few millimeters toward the crown. White and pale yellow stones become, briefly, very handsome gems. Blue sapphires naturally become a sort of muddy green, while light lilac stones become a rosy orange, near to pacparatum. Common gray star sapphires turn yellow brown. Natural sapphires show the change much more distinctly than the synthetics, though these too show changes which differ only in degree, not in hue. Prolonged exposure to sunlight gradually causes a reversion to the original hue; though it is rather difficult to get the last trace of color out of the originally colorless stones without some application of heat. In the dark, the color appears to remain the same indefinitely. Pale yellow stones fade more slowly and color more deeply than stones which are originally white. The fading of natural yellow sapphires in the same sort of sun lamp exposure as that to which the x-ray colored stones were tested was tried, and they were found to fade only slightly, the fading being not at all comparable to the fading of the color induced by the x-ray. If a method could be found which would make this color more permanent, it would be of considerable commercial importance. As it is, the color might last for years if gems so tinted were used only occasionally and where they would not be exposed to sunshine. Naturally deep yellow stones should be carefully scrutinized and subjected to fading tests before purchase when the tubes become generally available. The ubiquity of the amber coloration, regardless of any other pigmentation, suggests that the discoloration under x-rays is caused by a constituent universally present, regardless of the other pigmentation, but since it is weakest in the purest synthetics, it is due to an impurity or structural defect not abundant in the synthetic material, but universally present in natural Ceylon stones. No fluorescence or phosphorescence was observed in the corundum gems during and after exposure, with the single exception of a pale salmon-colored stone which fluoresced a brilliant orange during the radiation. In other respects this stone did not differ from experience with the other sapphires. Blue Australian sapphires were unaffected. TABLE I
Synthetic Corundum The synthetic corundums of various colors showed varying responses to the irradiation. Blue stones showed a slight greening, or, like synthetic ruby, no change at all. A deep pink stone became deeper red in color and a light pink became a rich red amber, much like the color produced in natural stones. A green sapphire became a dirty amber hue, and a colorless stone turned slightly brown. The exposures of all these synthetics were longer than those given the natural stones, and ranged up to fifteen hours. However, as with the natural stones, the changes induced seem to take place quickly, and further irradiation has little effect. A white American boule was slightly yellowed where the beam fell. Tourmaline A number of stones of different colors were treated, but no marked results were obtained. In general there was a darkening of color but it was very slight. The darkening in a deep rose-purple stone was accomplished by a ten hour exposure, though the total effect may have been attained much earlier, while a green stone showed no change after eight and a half hours. A pale green gem, on the other hand, turned light yellow with a treatment lasting but one hour and twenty minutes. Quartz (a) Rock Crystal: It has been reported that quartz oscillators turn dark on exposure to x-rays, but in our experiments colorless quartz appeared quite resistant to color changes. A series of tests on a single prism face of a crystal showed appreciable discoloration only after an hour or more of exposure. In this substance, individual quartz crystals will undoubtedly vary, for Frondel, 1944, reports the development of banding parallel to growth faces in the irradiated plates and is also able to detect twinning by differential coloration. This was noted by us in a large crystal which was irradiated. In this connection, Frondel notes that "the sensitizing factor apparently depends on the growth history of the mother crystal." Similar banding was noted in fluorite, and it seems likely the explanation lies in a varying percentage of lattice defects caused by variations in conditions during crystal growth, rather than an impurity included during growth. The color can be removed by heating as are the other stone colorations, but Frondel reports that the effect on oscillation frequencies appears to be permanent below 175° C. (b) Amethyst: Violet stones seem to be relatively unaffected by irradiation, though in some there was a suggestion of darkening. Heated amethysts, so-called "topaz," which had been changed to brown, became much darker, the result of the imposition of a smokiness on the yellow brown coloration. Here, as in brown topaz we find the two colorations unrelated and independent. Natural citrine also became smoky in the treatment. (c) Other Quartz Varieties: Rose quartz became very dark under irradiation, as reported by Frondel. Chalcedony was weakly affected, and the banding was accentuated by variations in response. Scapolite Scapolites of two types were tested and showed similar responses, with some variation. A white scapolite cat's-eye from Burma became deep violet after a brief (10 minute) exposure to the radiation. Penetration was not deep and best results were obtained by exposing different areas of the stone in succession. The induced color was strongly dichroic, ranging from deep violet parallel to the c-axis to almost colorless across it, and across the parallel inclusions which make the "eye." Because of this strong dichroism, the coloration of the gem is less intense than might be expected. This color fades rapidly on moderate heating or on exposure to light, and, unlike the other stones treated, it fades in the dark. Within one month of the treatment, a very deeply colored stone had become almost white again, although it had been kept in the dark all of the time. Of all the stones tested, this showed the most marked fading with time alone; some of the others may have faded but certainly none was as noticeable. A transparent pale yellow stone from Brazil was similarly treated and this likewise changed to violet. A comparable stone was treated by exposure to radium by Mr. Grant Waite of Toronto and both acquired a pale violet color, with pronounced dichroism. The coloration of the yellow gems appears to be no more permanent, the radiated stone likewise faded rapidly on exposure to light, but it was less sensitive than the white Burmese gem. A bright orange fluorescence and phosphorescence was observed, continuing for some minutes after the exposure. Opal A Mexican opal was subjected to radiation in the hope that darkening of the clear material of the back might make the color stand out better, but no change was noted in the stone after twenty hours of treatment. Similarly negative results were obtained in the treatment of a fire opal from Mexico. Topaz Specimens of blue and white topaz became smoky brown on exposure. This color did not resemble any natural topaz coloration. It was easily removed by heat treatment. On the other hand, pale yellow and pale pink Brazilian topaz had a definite brownish coloration added, of a type which, if permanent, would greatly enhance the value of the stone. Eight hours of exposure to sunlight removed most of the induced color. Zircon Several stones were treated, including blue heat-treated zircons and natural brown ones. The heat-treated blue partly reverted to a brown stone, a layer about 1 mm. thick became very brown, while the brown crystals were unaffected. On exposure to sunlight, one irradiated stone became blue again. Diamond A few stones were treated, off-color yellows and browns having been selected to begin with. No particularly remarkable results were obtained and most of the diamonds appeared unaffected. One of a matched pair of yellow brown stones was treated and after fifteen hours of irradiation it appeared to be somewhat lighter in color than its mate. Another pale brown stone appeared to have darkened a little. Since these stones were so small, it was difficult to judge colors, and further work in this field might be advisable. Certainly any process which could reduce yellow in diamonds is worth investigating further, for if the change is temporary there is a danger of treatment just before a sale; and if permanent, worth considering as a gem treatment. Other Experiments Heating and chilling: A number of other experiments were tried, with the thought that extreme temperatures coupled with x-ray bombardment might produce more spectacular or more permanent results. No particularly interesting results were obtained; but further work along those lines might be profitable. Sapphires which were heated above 300° C. at the time of the exposure to, the radiation failed to color at all deeply. Below that temperature progressively deeper colorations down to 100° C., were obtained and below that the results were the same as at room temperature. Supercooled stones, immersed in liquid air just before treatment, were colored in the same way and to the same degree as a stone at room temperature. Electrical tests: Experiments with strong magnetic fields made no difference in the color of the sapphires: likewise with strong electrostatic fields. Apparent conductivity was noted in a strong electric field, a current of 5.5 microamperes being obtained. However, this was caused by ionization of the air between the high voltage plates and not by any conductivity of the gem, as was shown by a test run without the stone in place. Fading by light and/or heat: Tests for fading due to sunlight were made on an accelerated basis by using a Westinghouse Mazda-Type RS 275 W type sunlamp, the fading effect of which on treated stones at a six inch distance was found to be approximately equivalent in one hour to two hour's exposure to mid-summer midday sun, in the latitude of New York. An ultraviolet germicidal lamp, having very little visible or infra-red output, produced no perceptible fading. An hour's treatment under the sun lamp resulted in the removal of most of the induced color in the case of treated sapphires. Fading tests on natural yellow sapphires showed that under the same conditions of light as were found to be sufficient to fade irradiated stones, comparatively little fading took place. On the other hand, irradiated stones which were originally light yellow, rather than colorless, assumed a deeper hue and faded more slowly in light than the originally colorless stones. Heating to a temperature of 300° C. for five minutes was sufficient to drive the last vestige of color from the originally colorless stones, but did not do this to the naturally yellow stones though there was some fading. Prolonged x-ray treatment had no effect upon depth of color or rate of fading, variations were found to be due to differences in the individual stones. X-ray quality effects: It was desired to test the influence of different quality x-rays upon the colors; it having been noted that the bright green obtained in spodumene by the treatment described above seemed to be more intense than in the case of stones previously treated by other experimenters, whose crystals appear gray-green. A kunzite was exposed to irradiation from a Machlett Thermax tube (a conventional Pyrex glass, oil-immersed tube) for thirty minutes at 155 PKV and 8 MA, and the stone became about the same green as already noted, after about an hour required for the disappearance of the phosphorescence. However, the high voltage rays had a greater penetrating power and gave an even coloration to the spodumene. In the previous experiments high surface absorption of spodumene had been noted, when the phosphorescence after ten minutes treatment was seen to be very close to the surface. Probably the grayer green noted in stones colored some years ago, for example some seen in the Goldschmidt collection in Heidelberg a number of years ago, had partly reverted to their original hue, or were incompletely colored in the first place, retaining much of the inner lilac hue, which naturally reduced the richness of the new color. A pale yellow sapphire treated for ten minutes at 125 PKVX10 MA and followed thirty minutes of 150 PKVX8 MA became a rich amber, possibly somewhat darker than the color obtained with the AEG-50 tube. This again indicates greater penetration of the high voltage x-rays, producing a more uniform coloration throughout the entire mass of the larger stones. In the experiments with the Thermax tube, the specimens were laid directly against the plastic window in the shockproof tube enclosure, and approximately 8 cm. from the focal spot. Supplementary note: In the course of the heating experiments, an interesting color phenomenon was observed on a lilac sapphire. This stone was irradiated to produce an orange color. On heating to 300° C. it was observed to become a blue-green. As it cooled after treatment, the original color reappeared. This color change with temperature was found to be independent of the irradiation, it was a temperature phenomenon, much like that which has been described for gillespite (Lee, 1936) and which jewelers have noted in working with rubies. Further thermal experiments with this type of stone should prove interesting. ACKNOWLEDGMENTS The authors wish to express their appreciation to Mr. R. R. Machlett for making the new AEG-50 tube available for the tests; to Mr. H. M. Paskow for the loan of gem material and to Mr. Joseph D'Agostino for his cooperation in initiating the experiments. The authors also wish to express their appreciation to Machlett Laboratories, Springdale, Connecticut, for supplying the color plate. NOTES
1 For an introduction into the physicists' explanations of these phenomena,
consult
Mott & Gurney, Electronic Processes in Ionic Crystals, Oxford (1940). REFERENCES DEBOER, J. H., Electron Emission arid Absorption Phenomena. Cambridge (1935). DOELTER, C., Handbuch der Mineralchemie. Vol. 1-4 (1912). FRONDEL, CLIFFORD, Frequency adjustment of quartz oscillator-plates by x-rays. Reeves Sound Laboratories (July, 1944). LEE, O. IVAN, Reversible photosensitivity in hackmanite: Am. Mineral., 21, 771 (1936). MOTT AND GURNEY, Electronic Processes in Ionic Crystals. Oxford (1940). ROGERS, T. H., A high intensity source of long wave-length x-rays: Industrial Radiography, 4 (no. 3), 35-39 (1945-46).
[var:'startyear'='1947'] [Include:'footer.htm'] |