|
|
Volume 27, pages 219-229, 1942 GOLD CRYSTALS FROM THE SOUTHERN APPALACHIANS STEPHEN TABER, University of South Carolina, Columbia, South Carolina. ABSTRACT Euhedral gold crystals are extremely rare compared with the amount of the metal produced, and very few have been preserved. Well-formed rhombic dodecahedra are reported for the first time from the Southern Appalachians. The crystals were obtained from placer deposits in Greenville County, South Carolina. Poorly-developed crystals, dendritic forms and wire gold have been reported from Georgia. Euhedral crystals and filiform gold develop in open spaces, for gold is too soft and malleable to displace most other minerals. Also because of.these properties its crystalline structure is readily destroyed by impact or even by polishing, but the high mobility of the atoms makes recrystallization easy. Euhedral crystals are deposited from solutions occupying cavities. Wire-like forms of the native metals result when the material for growth is available in only one direction, the wires being pushed out into the cavities by the addition of atoms at their base from solutions occupying small pore spaces in the walls. INTRODUCTION Gold in well-formed crystals is extremely rare compared with the amount of the metal produced. The few euhedral crystals preserved in mineral collections have been recovered mostly from placer deposits though the softness and malleability of gold make destruction of form during transportation highly probable. Primitive methods of recovering gold from such deposits are more favorable for the discovery of crystals than the methods now generally employed in placer and lode mines. Also the intrinsic value of gold tends to prevent preservation of crystals that would be of scientific value. Gold has been mined in the Southern Appalachians from placer deposits and veins for more than 150 years, and over 1,622,000 troy ounces (50,500 Kg.) of the metal have been produced. Therefore, it is rather remarkable that euhedral crystals of gold have not been reported previously from this region, but the writer has found no reference to such crystals in the literature. Genth (1859, p. 254-255) reported gold from Spottsylvania County Va., "showing very distinctly one rhombohedron, scalenohedron and basal plane," but he states that "it is a coating on tetradymite and evidently a pseudomorph after it." Gold in irregular crystalline masse wires and dendritic forms has been found at the Loud mine in White County, Ga. (Blake, 1885, p. 584, and Jones, 1909, pp. 45, 207, 208 and, Pl. II, fig. 1.), but well formed crystals have not been reported. EUHEDRAL GOLD CRYSTALS FROM GREENVILLE COUNTY, S. C. In 1938 the writer found euhedral crystals in a sample of gold presented to him by Mr. S. N. Boozer who had recovered it by panning stream gravels in the northeastern part of Greenville County, S. C. The total weight of the sample is 1.3817 gms. In size the gold ranges from a small nugget of 0.1 gms. to fine dust-like particles. Most of the gold is angular to subangular, but the larger pieces are smoothed and flattened. Some pieces are cavernous, as though the gold had been deposited as an interstitial filling, partly surrounding older minerals. Rectangular cavities suggest deposition about pyrite crystals. Mineral inclusions are present and one piece of gold is magnetic because of included magnetite or ilmenite.
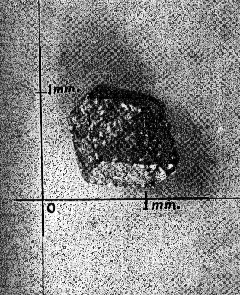
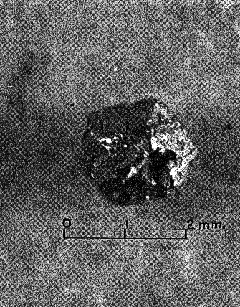
One well-formed crystal (Fig. 1), about 0.8 mm. in diameter and weighing 0.0039 gm. is a simple rhombic dodecahedron slightly flattened parallel to two faces and showing no evidence of modifying faces. The crystal faces are slightly pitted and the edges rounded by abrasion during transportation, but the luster is high. Some of the edges look as though they may have been slightly raised above the faces. A larger dodecahedron (Fig. 2), about 1.7 mm. in diameter and 0.0324 gm. in weight, is more scarred and rounded by abrasion, but it likewise shows no evidence of combinations with other isometric forms. This crystal contains a small but relatively deep cavity. Two small spheroidal grains present in the collection possibly represent well-rounded dodecahedra. An irregular shaped particle of gold shows a single, smooth, triangular face, and another shows a small square face, both having a high luster. These possibly represent the faces of other forms such as the octahedron and cube, but no conclusive evidence was m found of the presence of any crystal form other than the simple rhombic dodecahedron. The country rock in the area from which the gold crystals were obtained consists chiefly of gneisses and schists with intrusions of granite, and diabase. The gold occurs in small quartz stringers cutting sericite, schists, and also, with pyrite, as scattered impregnations in the schist. The area is in the upper Piedmont where deep weathering is prevalent under divides. These conditions are favorable for the concentration of` gold in stream beds, and, according to Sloan (1908, p. 32), limited placer deposits along a few streams in the area were worked successfully during the early days of mining, but efforts to recover gold from the veins were not encouraging. The appearance of the gold examined suggests that some of the particles have traveled farther than others but that most of it, including the euhedral crystals, has been transported for only a very short distance. GOLD CRYSTALS FROM GEORGIA Beautiful specimens of native gold from the Loud mine in White County, Ga., are preserved in the Museum of the Georgia Geological Survey, where they were examined recently by the writer. Part of the gold is in irregular masses or nuggets showing no evidence of crystal form, but the collection contains much gold in the form of strings of closely-crowded, small crystals with parallel orientation. Branching, resulting in dendritic, arborescent and reticulated forms is common. This type of crystallization is closely similar to that of gold from the White Bull mine in Oregon, described by Dana (1886, 132-138). Dana explains that the faces of the tiny "rhombohedrons" forming they strings are those of a tetragonal trisoctahedron elongated in the direction of the trigonal axis with suppression of other faces. Repeated twinning of {111}, together with branching at 60° results in the dendritic and reticulated forms. One specimen of gold quartz, probably the one shown by Jones (1909, Pl. II, fig. 1), contains a group of rounded grains or crystals, each 3 to 4 mm. in diameter, but the faces are so imperfect that the crystal form could not be determined with any degree of certainty. Cavernous faces seem to be present, but no smooth planes, as all edges are much rounded. No true wire gold is present in the collection. CRYSTAL FORMS AND STRUCTURE According to Dana's System of Mineralogy (Palache, Berman and Frondel, 1944, Vol. 1, p. 90-95) gold crystallizes in the hexoctahedral class of the isometric system, the commonest forms being the octahedron {111}, rhombic dodecahedron {011}, cube {001}, and tetragonal trisoctahedron or trapezohedron {113} . The tetrahexahedron {012} and hexoctahedron {124} are also reported; and several other forms, such as {014}, {013}, {025}, {118}, {114}, {112}, {223}, {3·5·11}, {237), {123} and {345}, are rare or uncertain. Shepard (1851, p. 231-232) describes a crystal of native gold from California having the shape of a pentagonal dodecahedron. He states that it is 2/5 inch in diameter, and presents distinct traces of the raised edges so characteristic of the native gold crystals of California, and which militates against the idea that these forms were produced in moulds left vacant from the decomposition of iron pyrites." This suggests that gold has the lower symmetry of the diploidal class, but gold crystals with cubic faces showing the characteristic striations of this class have not been reported.. Evidence from many crystals and from x-ray analyses shows conclusively that gold, and all the other metals of the gold group, crystallize in the hexoctahedral division and have a similar structure, the atoms being ganged in the simple face-centered cubic lattice with 4 atoms to theunit cell. If Shepard's crystal is not a pseudomorph it may be structurally tetrahexahedron with half of its faces missing. Most gold crystals reported are not simple forms but combinations of two or more forms, and some are highly complex. Simple cubes are very rare, though cubes combined with other forms, especially the octahedron, are relatively common. The octahedron is probably the commonest multiple form, and is said by Blake (1885, p. 576-577) to be the characteristic form in California, though most of the crystals are flattened parallel to {111} , or otherwise distorted. The rhombic dodecahedron seems to be more abundant in Australia an elsewhere. Professor Booth of the Philadelphia mint in a letter to Dana (1869, p. 8) states "that one lot of Australian gold worth about $4,000, submitted to him in 1853, consisted of grains from the size of a large pea to small sand, all of which were more or less perfect dodecalrons." However, some of the dodecahedra reported from Australia are not simple crystals of that form. Blake (1885, p. 575) states that "At Mount Ivor numerous small solid dodecahedrons were obtained retaining portions of the cubic faces, thus forming cubo-octahedrons," and Liversidge (1907, p. 143) shows that apparently simple gold crystals, such as well-formed rhombic dodecahedra from New South Wales, on etching yield ample evidence that they are not internally homogeneous but are in reality highly complex, and are composed of a number of individuals. These crystals may be pseudomorphs or they may be rhombic dodecahedra that have recrystallized. The tetrahexalledron, trapezohedron (tetragonal trisoctahedron), trigonal trisoctahedron, and hexoctahedron are rare as simple forms are not very common in combinations. Eubedral gold crystals apparently are produced only in cavities. Many minerals have such a strong tendency to develop crystal form that, in growing, they make room for themselves by displacing or by replacing most other minerals. Gold is so soft and malleable that it has little tendency to displace other minerals, and other minerals growing concurrently, or subsequently may easily exert sufficient pressure to destroy the forth of gold crystals. Gold crystals may grow around and thus enclose other minerals, and in the same way, other minerals enclose gold crystals. DISTORTED AND FILIFORM CRYSTALS Gold crystals, and especially octahedra, commonly show recessed cavernous faces and salient edges, a characteristic usually associated with relatively rapid growth and therefore, insufficient time for molecules to diffuse to the interior of faces. The dendritic, arborescent and reticulated forms are also commonly associated with rapid growth. Gold is deposited from solutions containing very small amounts of the metal as compared with the other vein-forming minerals present, but it is very easily precipitated from solution. Therefore a dilute gold solution maybe supersaturated with respect to gold crystals. Some writers have referred to the thread-like arborescent forms, made up of small crystals closely crowded upon each other, as "wire gold," but it would seem preferable to reserve this term for the smooth-surfaced wire-like forms of the metal. Silver occurs in this form much more commonly than gold. In some specimens several small wires gradually merge to form a single larger wire. They do not branch off at an abrupt angle as most of the dendritic or arborescent specimens. The wires are commonly curved and in some cases form a tangled mass of fine wires that more or less fill small cavities. The resemblance of the filiform varieties of the metals to the hair-like crystals of epsomite, chalcanthite and alunite that grow out from the walls of caves and that have been produced experimentally by the writer, suggest that the metallic wires have been formed in a similar manner. According to this hypothesis the wires are pushed out into cavities through the addition of molecules at their base from solutions occupying small pore spaces in the wall. The wire-like form is due to the fact that material for growth was available only at their base. If the depositing solutions were in contact with the wires elsewhere some crystal faces should be formed. RECRYSTALLIZATION OF GOLD Because of its softness and malleability, the crystalline structure of gold is easily destroyed. Beating of pure gold foil makes the metal harder and less plastic, and Beilby (1905, p. 223) has shown that this condition is accompanied by the disappearance of all outward traces of crystalline structure. The complete destruction of the crystalline units takes place only at the surface. "By carefully etching the surface in stages by means of chlorine water or aqua regia the successive layers below the surface were disclosed. The surface itself was vitreous. Beneath this was a layer of minute granules, and lower still the distorted and broken-up remains of crystalline lamellae and grains were embedded in a vitreous and granular matrix. The vitreous-looking surface layer represents the final stage in the passage from soft to hard, from crystalline to amorphous. By heating the beaten foil its softness was restored, and, on etching the annealed metal, it was found that the crystalline structure also was fully restored." Beilby (190,5, p. 222) states that "during polishing the disturbed surface film behaves exactly like a liquid under the influence of surface tension." Bragg (1914, p. 355-360) found that the outer surface of copper crystals, which have been much battered and distorted, retains little of the regular crystalline arrangement. Attempts to grind crystal faces artificially also destroyed the crystalline character of the surface and so prevented the reflection of x-rays from the face. Faces were therefore prepared for x-ray spectrographic work by dissolving the surface with acid. The recrystallization of amorphous gold that occurs quickly during annealing probably takes place slowly at relatively low temperatures, for gold atoms possess a high degree of mobility. Roberts-Austen (1896, p. 284-285), found that solid diffusion of gold into lead was relatively rapid at a temperature of 251° C. (70 mm. in 31 days), and that the rate of diffusion is still readily measurable at 100° C. Slow recrystallization following distortion and battering probably explains the rhombic dodecaedra composed of a number of individual crystals that were reported by Liversidge. REFERENCES BEILBY, G. T. (1905), Gold in science and industry: Smithsonian Inst. An. Rept., 215-234. BLAKE, W. P. (1885), The various forms in which gold occurs. Report of the Director of the Mint upon the production of the precious metals in the United States during the calendar year 1884, 573-597. BRAGG, W. L. (1914), The crystalline structure of copper: Phil. Mag. and J. of Sci., (1),a 28,355-360. DANA, E. S. (1886), On the crystallization of gold: Am. Jour. Sci., (3) XXXII, 132-138. DANA, J. D. (1869), A System of Mineralogy, 5 Ed., xlviii+827 pp. New York. GENTH, F. A. (1859), Contribution to mineralogy: Am. Jour. Sci., 2nd Ser., 28, 246-255. JONES, S. P. (1909), Second report on the gold deposit of Georgia: Geol. Surv. Ga. Bull. 19,: 283 pp. LIVERSIDGE, ARCHIBALD (1907), Jour. Roy. Soc. N.S.W., vol. XLI. Referred to by J. Malcom Maclaren on p. 84 of Gold: its geological occurrence and geographical distribution: London (1908), xxiii+687 pp. PALACHE, BERMAN, AND FRONDEL, (1944), Dana's System of Mineralogy, 7th Ed., vol. 1a, 834 pp. New York. ROBERTS-AUSTEN, W. C. (1896), On the diffusion of metals: Roy. Soc. London, Proc., LIX,281-285. SHEPARD, C. U. (1851), Large crystal of gold from California: Am. Assoc. Adv. Sci., Proc. VI, 231-232. SLOAN, EARLE (1908), Catalogue of the mineral localities of South Carolina: S. C. Geol. Surv, Ser. IV., Bull. No 2, 505 pp. [var:'startyear'='1942'] [Include:'footer.htm'] |