|
|
Volume 69, pages 413-439, 1984
The fluids in salt 1 EDWIN ROEDDER U. S. Geological Survey Abstract The fluids in salt have been used as sources of information on the geological events leading to the formation of the enclosing salt beds, and the subsequent changes to which these beds have been exposed. In recent years, however, consideration of salt beds or domes as possible sites for long-term nuclear waste repositories has added new significance to the study of such fluids. This paper reviews the current status of the study of the types of fluid present in salt, their origin and evolution, and their significance to understanding the geological processes that have occurred. These studies are pertinent to the engineering design of a nuclear waste storage site in salt in that they tell us what might happen in the future. The fluids in salt also introduce problems in the engineering design of a safe nuclear waste installation that must be carefully evaluated at each suggested site. Contents Introduction General nature and significance of the fluids in salt Simple microscopy of fluid inclusions in salt Primary fluid inclusions in primary bedded salt General features of evaporite basin formation Normal crystallization of salt Displacive salt Diurnal variation in inclusion density Primary fluid inclusions from dissolution and crystallization of bedded salt Dissolution (and recrystallization) processes on the salt basin floor Dissolution (and recrystallization) processes at some later time Fluid inclusions in domal salt Liquid inclusions Gas inclusions-"popping salt" Salt deformation effects Quantitative measurements of inclusions and their use Geothermometry-geobarometry Low temperature microthermometry Amount and nature of H2O present Amount and nature of gases present Eh and pH determinations on inclusion fluids Solutes present in aqueous fluid inclusion Isotopic signatures of H and O in inclusion fluids Significance of inclusion data to the nuclear waste problem Migration of inclusions in a thermal gradient Composition of the fluid inclusions Conclusions
Introduction In the early years of the development and operation of nuclear reactors in the United States, the military urgency was too great to permit much consideration of the ultimate disposal of the radioactive wastes that were generated. The major part of the radioactivity was kept in the form of high level wastes ("HLW"), consisting of liquids or slurries having a wide range of bulk and isotopic compositions (depending on the reactor type and the fuel reprocessing procedures used), and containing ~0.1 g mixed fission products per liter. The resultant radioactivity was generally in the range ~100 curies per liter (see Pines, 1978, for a general review). These wastes were placed in interim storage in shallowly buried tank farms. As such metal tanks have a life expectancy of only tens of years, a permanent storage site was needed. The length of time that these wastes must be kept out of the biosphere involves many factors and will vary with the specific reactor and waste type, but the general nature of the problem can be judged from calculations by Pigford and Choi (1976) summarizing the decrease with time of the biological hazard of the various components in one-year-old wastes from a given reactor operation (Fig. 1). The biological hazard is a complex function that is difficult to quantify meaningfully. In these calculations, it is given as ingestion hazard in units of cubic meters of water, per gigawatt-year of operation, needed to dilute the one-year-old or older wastes to the maximum acceptable levels for public drinking water. Note particularly that although the fission products are the overwhelming contributor to the hazard in the first years, their radioactivity decreases rapidly by ~1000 years, after which the small amounts of transuranic isotopes, particularly 241Am, 243Am, and 239Pu, and the increasing amounts of 226Ra, 228Ra, and other nuclides in their decay chains, take over to cause the hazard to stay relatively constant out to several million years. Finally, the decay of the long-lived iodine isotope 129I (half life 17 m.y.), which is relatively abundant as it is formed in ~1 percent of all uranium fissions, leads to the dropoff in hazard after 10 m.y. Eventually the biological hazard should become constant, as a result of non-radioactive constituents such as nitrate, fluoride, mercury and other heavy metals, etc. Where on the earth can these wastes be safely disposed of? In 1955 the National Academy of Sciences held an historic meeting at Princeton University (National Academy of Sciences-National Research Council, 1957; also 1966, 1970) to discuss this problem. The most limiting requirement for any such site was that it be dry, and stay dry for a long period, to keep the radioactivity out of potable waters. The most vivid recollections I have of that meeting pertained to the discussions of the possibilities of using salt deposits. Several of the attendees indicated that salt mines were the driest of all mines that they knew, and that obviously salt deposits must have been dry for a long time in the past; if not, they would have been dissolved in the intervening hundreds of millions of years. In the following 29 years salt deposits have been studied off and on as one of the possible geologic environments for nuclear waste storage. We now know that some salt is not quite as dry as first thought. However, a recent National Academy of Sciences study (National Academy of Sciences, 1983) indicates that salt beds and domes are still likely candidates for nuclear waste storage sites; hence the fluids in salt are of some importance. In addition, the study of the fluids in salt provides insight into paleoenvironments and the evolution of natural brines, and hence may also be pertinent to the problems of the origin and occurrence of petroleum.
General nature and significance of the fluids in salt The fluids in salt consist of two very different types that might be termed "external" and "internal." "External" fluids consist of incursions of outside waters, as have taken place in numerous salt mines in the past. An air-filled cavity far below the water table is basically unstable; those in salt are especially subject to flooding, as the flow of water through any leak dissolves salt and opens the passage to more flow. The recent flooding of the salt mine at Jefferson Island salt dome, Louisiana (Groat, 1981) apparently started from a drilling accident, but many other mines have filled, some catastrophically, generally after starting with natural leaks of unknown source (e.g., Commission, 1977; Knauth and Kumar, 1983; see also references in Baar, 1977). Such "fluids in salt" (from external sources), although important in nuclear waste storage, will not be discussed further in this paper. The "internal" fluids are those that are essentially inherent to the salt, although they may have originally come from external sources. They include the various types of fluid inclusions, and since radioactive waste will cause heating and decomposition of at least some hydrous minerals, they also include water in hydrous minerals in the beds. These fluids are significant to nuclear waste disposal in three ways: (1) Their occurrence, nature, amount, and behavior are important in both short-term site engineering and in long-term site safety. (2) Their occurrence and nature help in understanding the geologic processes that led to the formation of the salt deposit. This understanding in turn can be of help in both site selection, and in exploration for salt or potash deposits. (3) Their occurrence and nature help in understanding the long-term geological processes that have affected the deposit since it was formed. When a geologist is asked to predict the future of a nuclear waste storage site for periods such as are indicated in Figure 1, he must consider the two main classes of geologic processes - catastrophic and uniformitarian. The catastrophic include such rapid events as faulting, volcanic eruption, floods, and meteor impact. The uniformitarian include the more gradual effects such as climatic change and erosion. In both classes of events, the past record provides perhaps the best evidence for the probable events in the future. Any information that may be gleaned from the fluids in salt as to the processes that have occurred in the past is therefore pertinent to predicting the future. Simple microscopy of fluid inclusions in salt Primary fluid inclusions in primary bedded salt The ease with which salt recrystallizes causes some semantic problems in fluid inclusion nomenclature that may interfere in understanding of the processes involved. I will use the term primary bedded salt here to refer to any salt that has retained the original halite crystals which formed in the original evaporite environment. Fluid inclusions that were trapped during that original crystallization are thus primary fluid inclusions in primary bedded salt.2 General features of evaporite basin formation. Before trying to understand the origin of any specific inclusions it is useful to review the formation of evaporite deposits. Figure 2 is a sketch showing the generally accepted concepts (e.g., Hanford, 1981) of evaporation from a partly closed basin with limited influx of seawater. Meteoric water also enters the basin as runoff from the adjoining land mass, carrying detritus. As such fresh water is much lower in density than the evaporated brines, it can float out for long distances over the saline surface. Meteoric water can also enter the saline basin directly as rain, as subsurface groundwater springs, and any meteoric water falling on coastal sabkahs can dissolve surface salts and carry them basinward, either as surface or subsurface waters. During evaporation, the precipitation of NaCl or other phases will change the solute composition of the remaining fluid, and the evaporation itself will change the hydrogen and oxygen isotopic composition of the remaining fluid.
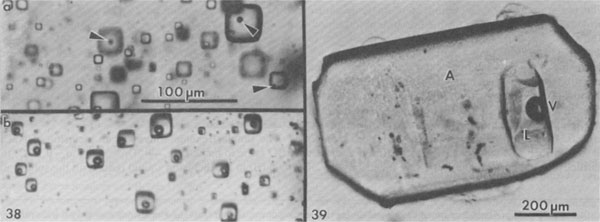
Normal crystallization of salt. Numerous studies have been made of the sequence and nature of halite and other precipitates formed under such conditions (e.g., Valiashko, 1951; Dellwig, 1955; Holser, 1979; Shearman, 1970, 1978; Wardlaw and Schwerdtner, 1966). The crystallization of halite has been studied in most detail, and two major types of primary salt crystals are described by these various workers. Evaporation at the surface of the brine can cause the nucleation there of small crystals hanging by surface tension on the air-brine interface. These grow at the edges by additions from the surrounding supersaturated brine, and hence form thin square tablets. The tablets usually have depressed centers, as a result of floating lower and lower as they grow heavier, and hence are called hopper crystals. They may join to form floating rafts, but eventually grow too heavy to hang, or are sunk by waves. On the bottom of the basin these sunken hoppers or other nuclei continue to grow as density currents bring down supersaturated surface brine. Growth competition between variously oriented nuclei favors those with the fastest growth direction pointing upward toward the source of new material. This competitive growth process has been well documented by Wardlaw and Schwerdtner (1966) for Devonian salt beds in Canada and by Shearman (1970) for Recent salt in Baja California. In salt this fastest growth direction is perpendicular to (111), i.e., the cube corners. The growth stages are normally outlined by zones of fluid inclusions, arranged parallel to the growing cube faces, and as the bottom is covered with cube corners at this stage, a vertical section through such salt will show a chevron-like pattern of fluid inclusions, as seen in Figure 3A. These features are best seen in transmitted light, in doubly-polished plates 2 to 10 mm thick, cut perpendicular to bedding. Such salt is called chevron salt, and the cubic planes of inclusions in it, like those in hopper salt, are believed to be primary inclusions in primary bedded halite. The individual crystals may be only a few millimeters in width, but as much as several centimeters in length. The inclusions are generally small slightly rounded negative cubes and may be exceedingly abundant; 109/cm3 is common and 1010/cm3 is not unusual (Fig. 4). Even at 1010/cm3, if they average under 1 µm in size, as is common, the salt may contain only 0.l wt.% of inclusion fluid. The banding in such salt is a result of differences in the abundance of fluid inclusions (Fig. 5); in reflected light, the densely packed bands appear white, and in transmitted light they may be almost opaque unless the plate is thin. Only rarely do the bands show curvature (Fig. 6), presumably from growth on a corroded substrate.
Salt crystals, like most substances in the laboratory and in nature, trap more fluid inclusions at high rather than at low growth rates. Other variables may be involved occasionally, but I believe that the banding in inclusion density so commonly seen in salt is essentially a qualitative record of crystal growth rates. An additional feature of interest is the common difference in the abundance of inclusions in salt that crystallized near the cube edge compared with that from the centers of the cube faces. In many inclusion-rich natural and synthetic salt crystals, a thin sheet of essentially inclusion-free salt may mark the growth path of the cube edge (Fig. 7). Displacive salt. In addition to free crystallization into a fluid, some salt has grown as 1-2 cm crystals suspended in soft mud or silt interlayers (Schreiber and Hsü, 1980). Such crystals, called displacive salt as they have grown by pushing the silt aside, may contain large inclusions of silt, and occasional fluid inclusions (some with silt). The porosity of the silt is also commonly filled with halite cement. It seems likely that the displacive salt cubes grew while the enclosing silt was still soft, but the formation of the salt cement could have occurred at any later time. Diurnal variation in inclusion density. Doubly polished plates of some salt from several cores taken in the Palo Duro basin, Texas, show a regular banding within some 0.5 to l.5 cm halite crystals. The banding consists of a regular alternation of clear and dark-gray salt, arranged parallel to the cube face (100). The dark-gray color arises from numerous, minute, slightly rounded, cubic fluid inclusions (Fig. 8). Although a few of these inclusions are 30 µm on an edge, or even larger, most are less than a few micrometers, or even less than 1 µm. The gray salt contains as many as 109 to 1010 inclusions per cm3. Because the clear bands are much thinner than the dark-gray bands, in effect they divide an otherwise uniform dark-gray salt into separate compartments and, hence, are here called septa. They are essentially free of inclusions and average about 20 µm wide in most crystals; in a few, they are ~100 µm wide. On either side of the clear zone, the density of inclusions increases abruptly. The spacing between septa (perpendicular to the banding) generally is rather uniform within a given crystal but differs from one sample to another. The average spacing for septa in individual crystals ranges from 0.40 to 0.85 mm. Some individual crystals show 10 or more bands. The fluid inclusions between the septa are commonly arranged in parallel bands (Fig. 8c) and some larger (~200 µm) cubic inclusions exhibit one very flat cube face directly against the septum. In contrast, the other five cube faces, against dark-gray salt, are rounded and only subcubic in outline. In some of the banded salt crystals, particularly the elongated ones, two sets of such clear septa join at a sharp 90° angle along what is more or less the center line in the long direction through the crystal. An additional similar clear septum is visible connecting these apices, effectively bisecting the 90° angle (that is, approximately in the plane of the dodecahedron [110]; see arrows in Figs. 8a and 8b). Both types of clear septa may be invisible unless the sample is tipped for viewing parallel to the plane of the septa. No petrofabric studies have been made, but the available plates indicate that the elongated banded crystals, and the nested rows of apices, are roughly subparallel and originally pointed more or less vertically in the salt bed. Although much of the salt consists of irregular, completely clear areas, most crystals of halite in these beds have at least some irregular areas of gray, inclusion-rich salt, similar to that between the septa. Because the boundary between the gray areas and these masses of clear salt is a sharp curving surface (even within a single crystal), which cuts across the banding of the gray salt, I believe that the inclusion-rich salt has been dissolved and recrystallized to yield the areas of clear salt (see next section).
Fig. 4. Dense cloud of primary fluid inclusions in single crystal of primary chevron salt from Palo Duro basin, Texas. Fig. 5. Typical banded array of primary fluid inclusions in primary chevron salt crystal from Delaware basin, New Mexico. Fig. 6. Atypical banded array of primary fluid inclusions in primary chevron salt crystal from Palo Duro basin, Texas. The curving bands of many minute inclusions probably represent growth stages on a slightly corroded substrate. Fig. 7. Typical NaCl crystal which grew as a cube with dense concentrations of minute fluid inclusions at the centers of each (100) face (opaque) and essentially inclusion-free corners. Synthetic crystal (~1 mm).
The distribution of the inclusions in the banded salt crystals indicates that they are almost certainly primary; that is, they must have formed during euhedral growth of the host crystals from a solution. If so, these inclusions presumably contain samples of the fluids from which this salt crystallized during the Permian. The depth at which chevron growth may have occurred has been a subject of much discussion (Dellwig, 1955; Wardlaw and Schwerdtner, 1966; Arthurton, 1973; Friedman, 1978), and the banding described here bears on that discussion. Evaporation at the surface will yield a dense, supersaturated solution, which is gravitationally unstable and will sink in a pattern of convection cells, or "stream tubes" (Bradley, 1965). On contact with the halite crystals at the bottom, the excess salt in solution will crystallize. In nearly all crystal-growth studies, a high degree of supersaturation results in fast growth and imperfect crystals containing many inclusions, and slow growth yields more nearly perfect crystal growth.
Alternation of inclusion-rich and inclusion-poor zones forms the chevron texture in the many salt deposits where it has been described, but this alternation is generally shown to be highly irregular, both in spacing of the bands and in the density of inclusions in each band. The highly regular alternation of inclusion-rich and inclusion-free salt within the Palo Duro crystals suggests a regular alternation of the degree of supersaturation of the liquid, as might be expected to occur diurnally; the inclusion-rich salt probably crystallized during the day, and the clear septa at night, as proposed by Holser (1979). A crude upper bound can be placed on the amount of evaporation this crystallization might have entailed. The sea bottom might have had relatively few such crystals protruding into the liquid, or a complete carpet of them. If a carpet of crystals existed, the maximum amount of precipitation of salt during a given day would be a layer ~0.85 mm thick over the entire bottom. This would correspond to the total evaporation of water from about 3 to 6 mm of salt-saturated seawater in that day. The actual amount of evaporation could be considerably less than this if the precipitation was not over the entire bottom. Similar evaporation rates have been recorded before, although they were based on measurements of presumed annual cycles (Dellwig, 1955; Wardlaw and Schwerdtner, 1966). If the previously described mechanism is correct, it also places some constraints on the depth of the water under which this salt crystallized. The deeper the water, the more mixing will occur as the currents of dense, supersaturated fluid flow downward, and the less sharp will be the changes in conditions at the bottom throughout a given cycle. Bradley (1965) has shown experimentally that vertical density currents ("stream tubes") tend to break up after a few centimeters of movement. If we assume that these bands were deposited during a 24-hour cycle, we eventually should be able to place some limits on the water depth, using estimates of the density contrasts and temperature gradients, expected turbulence and diffusion rates, and the thickness of the individual bands. At present, it appears highly unlikely that periodic density currents from a few millimeters of denser surface water could travel down vertically more than a few feet without mixing (Bradley, 1965), thus losing periodicity in the precipitated salt. Many bedded halite deposits show a regular banding or lamination parallel to the bedding (Dellwig, 1955; Wardlaw and Schwerdtner, 1966; Arthurton, 1973; Nurmi and Friedman, 1977). These laminae, which have been assumed to reflect annual variations in the conditions of deposition, are defined by differences in NaCl color or grain size and particularly by a band of calcite or anhydrite (or gypsum) grains for each cycle. For example, in the Michigan Basin, the calcite-anhydrite layers are 2.5 to 30 cm thick, and the intervening salt layers are 2.5 to 150 cm thick, yielding 5 to 180 cm of bed per cycle (Nurmi and Friedman, 1977). This sequence of change in composition of the material being precipitated agrees with the sequence of phases, if not with the quantities, that would be expected during the evaporation of a given unit of seawater (see Dean, 1978; Friedman, 1978). The periodicity of the salt crystals I describe here, however, occurred while a single phase (NaCl) was being precipitated, and each cycle represents the deposition of two to three orders of magnitude less material. Primary fluid inclusions from dissolution and recrystallization of bedded salt Much bedded and most dome salt does not show evidence of the hopper or chevron growth described above, but instead consists of clear, relatively equant salt crystals, with smooth grain boundaries and frequent 120° triple junctions. Such a recrystallized, essentially "metamorphic" rock is the end product of a long series of relatively low-temperature diagenetic processes. These may take place isochemically, in which case any new fluid inclusions will have the same composition as those in the original primary salt, or they may involve loss and/or gain of fluids. Fluid inclusions are trapped during some of these diagenetic processes, and can provide some information on the processes, but it is difficult to establish any time frame - or even the sequence - of the various processes. Dissolution (and recrystallization) processes on the salt basin floor. Salt beds frequently show evidence of partial dissolution, of several types. As seen in Figure 3B, the tops of the projecting crystals may be dissolved away, presumably by a layer of undersaturated seawater or fresh meteoric water. Renewed crystallization of salt can occur on the crystals making up the pavement, or on new nuclei, or both (Fig. 3C). In addition to planing off the crystal tops, many salt beds show evidence of extensive solution between the crystals (Fig. 3B). These solution cavities may subsequently be partly filled with mud, but commonly the open spaces are eventually filled with coarsely crystalline salt, usually growing as extensions of the crystals comprising the walls. The origin(s) of such clear salt is important, as many of the larger fluid inclusions, most amenable to study, occur in such material. If this cavity-filling salt can be shown to have been planed off along with the chevron crystals, it is obviously early. However, it is common to find such cavity-filling salt as pipe-like masses crosscutting many centimeters (or decimeters) of beds, suggesting that some of the cavities may be formed later, though perhaps still near to the basin floor or even while the salt bed was above the water table. The specific mechanism(s) yielding the solution cavities are not obvious; all that is obvious is that undersaturated fluids were involved. Of the many possible scenarios, the three most likely are: (1) The beds may have been exposed subaerially by desiccation of the salt basin; this would permit brines from salt dissolution in rain (or dew) to percolate down and toward the basin. (2) If the salt is not at the bottom of the basin, simple flooding with unsaturated water would similarly permit percolation of dense brines down and basinward. (3) Entry of unsaturated groundwater from below, driven by pressure from a higher-level recharge area (Fig. 2), would dissolve channels. Very detailed field observations, preferably on something more than a drill core, present the best possibility of understanding the process(s). The evidence of dissolution is relatively unambiguous. Earlier structures, outlined by growth planes of inclusion-rich chevron salt, are truncated by smooth, undulatory surfaces beyond which the salt is clear, even though all is one single crystal (Figs. 9a,b). An additional unanswered question is the cause of the change from undersaturated to oversaturated fluids, thus forming the clear salt overgrowths. Dissolution (and recrystallization) processes at some later time. Examination of much bedded rock salt in thin section reveals scant evidence of the primary depositional features described above. Some salt crystals may contain a few small areas of chevron banded material (Fig. 10); at low magnification these areas may appear as small gray wisps randomly scattered through large clear areas, and commonly make up <1% of the sample. Much rock salt now consists of clear, uniform, equigranular crystals, usually outlined by minute fluid inclusions (Fig. l1).3 A few such crystals may show parallel bands of minute fluid inclusions in their centers, presumably inherited from the original basin crystallization textures (Fig. 12). The remainder of the crystal consists of a clear "overgrowth" (actually a recrystallization), presumably formed during the development of the "metamorphic" texture. Some such "recrystallization" obviously occurred on the floor of the salt basin (e.g., Fig. 3), and other recrystallization probably occurred under deep burial, but the distinction between these two is difficult. We may have to use the inclusion data to make the distinction, rather than the reverse. Thus if the inclusions contain compressed gases, they cannot be from trapping at the surface. Clear recrystallized salt generally has few inclusions, but they may be large, and the larger the host salt crystal, the larger the inclusions. The smaller inclusions (< 1 mm) tend to be cubic in shape, but very large inclusions may be rather irregular (Fig. 13a,b). A small, moveable shrinkage bubble, generally <= 1 vol.% (Fig. 14), is found in most of the larger inclusions, but is seldom seen in the smaller ones. Some of the larger inclusions may contain much more than 1 vol.% of bubble; many of these larger bubbles merely signify relatively recent leakage, possibly after collection, along cracks that are sometimes visible (Fig. 14). In others, the "leakage" has occurred at depth, since the bubble consists of gas at many atmospheres pressure (Fig. 15). In still others, the evidence is clear that a separate, immiscible dense CO2 fluid phase under considerable pressure was present at the time of formation of the inclusion (i.e., during the recrystallization of the host salt; Fig. 16). The fluid in such inclusions in recrystallized bedded salt is generally a water solution of various ions, saturated with respect to NaCl. Most do not show any daughter crystals, i.e., crystals that have formed from the fluid after trapping. Solid crystals are not rare in such inclusions, but most are extremely irregular in their distribution and represent accidental solid inclusions of phases that were present at the time of trapping of the fluid inclusion, such as other precipitates or detrital silt (Fig. 17). Small amounts of solid organic matter are sometimes seen adhering to the liquid-vapor interface, as well as tiny droplets of an immiscible liquid having n > brine. These droplets are presumably petroleum; if several inclusions show the same volume percentage of petroleum, they may be a true daughter phase, and stem from changes with temperature in the solubility of petroleum in brines (Price, 1976). Fossil halophilic (salt-loving) bacteria and algae may be present and should be looked for. True daughter crystals are not uncommon, however, in salt beds that are associated with K deposits. In such environments, the large concentrations of K and Mg in the brines, and significant temperature coefficients of solubility, can result in the formation of true daughter minerals on cooling from the temperature of trapping (i.e., of diagenesis?). Any of the normal evaporite minerals might be found thus, but carnallite is perhaps most common (Fig. 18). The occurrence of a potassium mineral such as carnallite as a daughter phase may indicate that the host salt formed from fluids that had evaporated at least to that stage, even though no potash beds have been found. On the other hand, such K-rich fluids may represent residual bitterns from the crystallization of potash beds, or solutions formed by their later dissolution, that have migrated into these salt beds. Regardless of the specific mechanism of formation, K-bearing daughter crystals in inclusions in salt provide a useful tool for the exploration for K deposits in that evaporite basin. Salt from the Palo Duro basin, Texas, has been found to include a manganese oxide phase, whose origin is still in doubt; it may well be a primary precipitate, formed with the salt, that survived through some crystallization. Although it does not seem to be a daughter phase, it is usually associated rather closely with the fluid inclusions in this salt. It occurs in quantitatively miniscule amounts, but the possibility exists that any individual phase in the salt may provide insight into the environment of formation or the latter diagenesis of the beds. Manganese oxide occurs in two crystal habits in these samples, as fibers and as "tubes"; they may both be the same phase.4 The fibers are seldom as much as 10 µm thick, and many are < 1 µm, but single fibers may be traced for a millimeter or more in the host salt crystal. Some branch, and occasionally spiral, and split ends are common. Many of the fibers are found in the fluid of inclusions, and penetrating the walls (Fig. 19). The fibers are dark yellow brown, very slightly pleochroic, highly birefringent and length-fast with parallel extinction. SEM studies show major Mn and minor K, and X-ray diffraction patterns indicate the cryptomelane structure (K(Mn4+Mn2+)8O16). The "tubes" seem to be a truly novel mineralogical phenomenon. They are smooth, straight, uniform open-ended hollow cylinders (Fig. 20), with square ends and thin walls (1-2 µm). Some are 200-300,um in length and only 10 µm in diameter; others are larger in diameter than in length. The optical properties are like those of the fibers, and each tube behaves optically as a single crystal. SEM analysis showed major Mn and minor K. Most of the tubes occur in fluid inclusions in recrystallized salt, but several were found in chevron salt.
Fig. 9a. A dense cloud of primary inclusions in single crystal of primary halite, similar to Fig. 4 but from the Delaware basin, New Mexico, showing some primary banding (arrow), and sharp but curving contact with crystallographically parallel but almost clear recrystallized salt (at top). Presumably the curving contact is from dissolution of the earlier inclusion-rich salt, followed by later, slow, almost inclusion-free growth. From Roedder and Belkin (1979a). Fig. 9b. Bands of primary inclusions in primary halite, from Palo Duro basin, Texas, that have been truncated by later solution and growth of new inclusion-free salt. Entire field of view is a single halite crystal. Fig. 10. Area of primary inclusions in primary halite, from Palo Duro basin, Texas, surrounded by clear salt. Entire field of view is a single halite crystal. Fig. 11. Intercrystalline fluid inclusions (gas) on interfaces outlining 120° junction between several recrystallized salt crystals in core from ERDA No. 9 borehole, Waste Isolation Pilot Plant site, Carlsbad, New Mexico. From Roedder and Belkin (1979a). Fig. 12. Halite crystal in rock salt from bedded salt from the Delaware basin, New Mexico, showing a core containing primary fluid inclusions in primary chevron salt, and a rim of relatively inclusion-free recrystallized salt. Fig. 13. Most inclusions in halite are regular simple cubic cavities (Figs. 4 and 5), but some other shapes are found. (a) Large, irregular primary inclusions in recrystallized salt from Delaware basin, New Mexico. (b) Cube modified by the octahedron and dodecahedron, in recrystallized salt from Palo Duro basin, Texas. Fig. 14, 15. Pairs of primary inclusions in single crystals of recrystallized bedded salt from Delaware basin, New Mexico. In each Fig., inclusion on left has small bubble (~1 vol.%), typical of the inclusions in this sample, but inclusion on right has a large bubble. In Fig. 14, this bubble is air, from leakage along recent(?) crack (arrow). In Fig. 15, the inclusion has presumably been opened at some later time along a crack (arrow) and part of the original fluid replaced with gas under several atmospheres pressure. From Roedder and Belkin (1977).
Fluid inclusions in domal salt Salt from salt domes normally shows only "metamorphic" textures, and no trace of the original depositional features such as chevron salt, whereas salt from the less-severely deformed salt anticlines may still have some such features preserved. In addition to the dramatic evidence of large scale complex isoclinal folds, faults, and a generally complete recrystallization, the salt crystals in some salt domes show strong deformation fabrics. Such deformed salt consists of parallel arrays of salt crystals that are 2 or 3 times as long as wide. What happened to the fluid inclusions (l to 2 vol.%) that were probably present in the original bedded salt before such deformation? Liquid inclusions. Most domal salt has fluid inclusions, but they may be exceedingly scarce and very erratically distributed. Most can only be assumed to be primary with respect to the salt in which they occur, but there is no way of knowing when (or how often) the host salt has recrystallized. The total amount of visible liquid may average one or two orders of magnitude less than that in bedded salt so apparently most of the fluid in the original fluid inclusions has been squeezed and kneeded out, probably by migrating along grain boundaries (Baes et al., 1983). The inclusions that are present are occasionally large and commonly contain an anhydrite crystal (Fig. 21). This is probably not a daughter crystal, but rather a solid inclusion. The association of the two is a result of preferential wetting; the lowest energy state seems to have the brine at the interface between anhydrite and salt (Fig. 22), and in some salt, the only visible fluid inclusions are tiny fillets between crystal of anhydrite (Fig. 23), or between solid inclusions of halite in some sylvite (Fig. 24). As in bedded salt, true daughter minerals in dome salt are more common if potassium salts are present (Fig. 25). Petroleum, trapped with the brine as immiscible droplets, is common in many domes (Figs. 26, 27).
Fig. 20. Primary fluid inclusion in single crystal of recrystallized bedded salt from Palo Duro basin showing two hollow cylindrical crystals of a manganese-bearing phase (possibly also cryptomelane; see Fig. 19). The axis of the small cylinder (arrow) is almost in the plane of the photograph. The fluid inclusion is a negative cube modified by curved faces of the octahedron (pebbly surface). See text.
Gas inclusions - "popping salt." Gas under pressure is occasionally found as isolated gas inclusions within single salt crystals (Figs. 16, 28), but in most dome salt that I have examined, at least some such gas is present as very small inclusions at the interface between salt and anhydrite, where the latter crystals are completely enclosed by a single salt crystal (Fig. 29). Similar inclusions are found in samples from salt anticlines, as at Asse, FRG (Roedder and Belkin, 1981). The pressure in the inclusions shown in Figure 29 obviously was not high enough to cause the host to break or decrepitate before photography, but a large number of such inclusions distributed through a mass of salt places the host under considerable stress. When a mine face is cut into such salt, it may spontaneously start to decrepitate into the mine opening; this relieves the confining pressure on the salt behind, so it continues to decrepitate. Such "blowouts" can suddenly throw thousands of tons of broken salt into the mine, and have killed miners (Belchic, 1961; Hoy et al., 1962; Thoma and Eckart, 1964). The presence of such "popping salt" can be detected qualitatively by the little popping sounds from underfoot, where the pressure of the shoe adds a little more stress to an already stressed fragment of salt. The cavity resulting from a blowout is rather amoeboid in shape, with smoothly curving walls. Only the more inclusion-rich salt decrepitates, and the concave curvatures of the walls are such that the resulting slight additional confining force from the concavity keeps the remaining salt from decrepitating further. One such cavity I saw in a Polish salt anticline opened out to a large and complex bifurcated chamber, all the contents of which had blown out through a very much smaller opening in the back (roof) of the mine. The composition of the gases in such salt is discussed below, but one simple qualitative test under the microscope is adequate to verify the existence of gas inclusions under pressure, even if they do not result in "popping." Simply dissolve the salt in water while watching under the microscope. A crude estimate of the gas pressures can sometimes be made from the amount of expansion (Roedder, 1970; see also Fig. 30). Sometimes when this test is used, liquid inclusions that appear to be ordinary brine are found to be liquified hydrocarbon gases (Fig. 31). Salt deformation effects. The salt in salt domes and salt anticlines has been deformed, and hence one might expect abundant evidence of secondary inclusions. Since salt is so plastic,5 particularly when moisture is present and temperatures are elevated, relatively few planes of obviously secondary inclusions are seen (Figs. 32, 33). The recrystallization of salt is so easy, however, that most of the inclusions now seen in dome salt may have originally been secondary inclusions, introduced during dome formation, and subsequently recrystallized and redistributed. Recrystallization of inclusions in salt is so fast that obvious examples of necking down in progress (e.g., Fig. 33) are almost certainly of relatively modern origin. In addition, salt can deform without the formation of obvious shear planes. I have found dome salt cleavage blocks that were optically clear but had curved cleavage surfaces; one had a curvature of ~30 cm radius. Under such conditions, a fluid inclusion can be stretched out by the deformation, particularly when anhydrite crystals are present to act as anchor points (Fig. 34). Moist polycrystalline halite and other evaporite minerals have been found to flow with effective viscosities as much as six orders of magnitude lower than the equivalent dry material (e.g., Talbot et al., 1982; Urai, 1983a,b). Furthermore, the presence of a liquid film between the grains greatly enhances recrystallization and can cause the trapping of new fluid inclusions (Urai, 1983a). Although no data are available on the quantity of fluid needed to achieve these physical effects, it is apparent that it need not be large, and it is possible that the opening of some inclusions by deformation of the host may be adequate to provide enough moisture. Quantitative measurements of inclusions and their use Geothermometry-geobarometry The most common usage of fluid inclusions in geological studies has been to use the temperature of homogenization (Th) of liquid and vapor inclusions as a geothermometer (and sometimes as a geobarometer). Some dome salts, in particular, have come up from salt beds that were at depths of 10 km or more. The geothermal gradient at the time would be difficult to estimate, since salt is so much more conductive than ordinary sediments, but even if the gradient were only 10°/km (Fig. 35), such salt must have been at temperatures of at least 150°C. Can the Th values for inclusions in such salt be used to establish these formation temperatures? Many homogenization measurements on salt have been published (see Roedder, 1968, for references), but I doubt their significance, since salt is plastic, particularly in the presence of water (Fig. 36). What would happen to a fluid inclusion, trapped at some higher temperature, during cooling as the host salt moves closer to the surface? Normally, a bubble would form inside the inclusion once the temperature had dropped to the boiling curve for such a brine. This bubble would contain low pressure steam, since the vapor pressure of saline fluids is low relative to that of pure water. But the external lithostatic load would be much greater than the internal steam pressure, and if the salt is plastic, it might collapse into the inclusion, eliminating the bubble. Figure 37 shows that such collapse of stressed salt surrounding an inclusion can occur even within minutes, and even at room temperature. In nature, the temperatures were higher, and the available time was as much as 1013 times greater.
Fig. 21. Fluid inclusion (presumably primary) in single crystal recrystallized halite (H) from Vacherie salt dome, Louisiana, containing a vapor bubble (v), and a striated anhydrite crystal (A) that is presumably an accidental solid inclusion. From Roedder and Belkin (1979b). Fig. 22. Fluid inclusion (presumably primary) in single crystal recrystallized halite (H) from Oakwood dome, East Texas, showing many anhydrite solid inclusions and small vapor bubble (v) in liquid (L). The liquid preferentially wets the anhydrite surface, and hence clung to these crystals as the inclusion changed shape. From Dix and Jackson (1982). Fig. 23. Group of anhydrite crystals with fillets of adhering brine (arrows), in halite (H) from Rayburn dome, Louisiana, photographed in strongly convergent light, at slightly different levels of focus. In normal collimated microscope lighting, these fillets are hidden in broad black shadows. From Roedder and Belkin (1979b). Fig. 24. Solid inclusions of halite (H), bridged by an aqueous inclusion (L), in sylvite (S) from the Permian El'tonskoye deposit, pre-Caspian basin, USSR. From Petrichenko (1977, his Fig. 99). An adjacent inclusion, out of focus, contains only gas. Fig. 25. Three-phase inclusion in sylvite (S) from the Stebnikskoye potash deposit, pre-Carpathian depression, USSR. From Petrichenko (1977, his Fig. 88). The cubic daughter mineral is halite (H), and the hexagonal one is carnallite (C), in brine (B). Fig. 26. Plane of secondary inclusions in halite crystal (H) from Week's dome, Louisiana, showing very small vapor bubble (V) and immiscible globule of oil (O) in brine (B). Note that the oil has an index of refraction near to that of the host salt. From Roedder and Belkin (1979b). Fig. 27. Inclusions of dark brown fluid, presumably oil, each with a vapor bubble (arrows) on surface of anhydrite crystal (A) embedded in a single crystal of halite (H) from Rayburn dome, Louisiana. From Roedder and Belkin (1979b). Fig. 28. Plane of secondary inclusions of low pressure organic gas (CH4?) in halite (H) from the Neogene Stebnikskoye potash deposit, USSR. From Petrichenko (1977, his Fig. 216). Fig. 29. Planes of high-pressure gas inclusions at the interface between included solid anhydrite crystals (A), and the single-crystal host halite crystals (H). From Roedder and Belkin (1979b). (a) From the Rayburn dome, Louisiana; (b) from the Winnfield dome, Louisiana.
Fig. 30. Two anhydrite crystals in single halite crystal (H) from Rayburn dome, Louisiana, during dissolution test for high-pressure gases. (a) The solution front is advancing parallel to plane of the photograph but has not yet intersected the inclusions. (b) Same field as (a) after solution front contacted these two crystals. Note large gas bubbles (arrows) formed from almost invisible high pressure gas inclusions. From Roedder and Belkin (1979b). Fig. 31. Inclusion of presumed liquified hydrocarbon (L), in crystal of halite (H) from Vacherie dome, Louisiana, during dissolution test for high-pressure gases. (a) Inclusion before solution front (approaching from above and approximately parallel with plane of photograph) intersects it. (b) Same field as (a) after intersection of solution front with inclusion. All liquid has vaporized and residual bubble fills the inclusion. Total apparent expansion of gas bubble 342-fold; actually the liquid flashed into gas when exposed to atmospheric pressure. From Roedder and Belkin (1979b). Fig. 32. Crystal of salt from Week's dome, Louisiana, oriented with cubic cleavage directions vertical, horizontal, and parallel with plane of the photograph showing a group of planes of secondary inclusions arranged approximately parallel to the dodecahedron (110), presumably from slippage. The individual shear planes are visible only at the end of the group (arrow), where the original cracks pinch out. From Roedder and Belkin (1979b). Fig. 33. Photomicrograph of a healed fracture (approximately parallel to (110)) in a halite crystal, showing nonuniform phase ratios caused at least in part by necking down of an original large, thin, flat, secondary inclusion into many smaller inclusions of lesser total surface area. The vapor bubbles formed before and during the necking down, so that individual inclusions now have a wide range of gas/liquid ratios (a, b, and c) or no gas at all (d). As the "gas" in the bubbles is merely water vapor in this sample, the composition of the fluid in all will be essentially the same, but the homogenization temperatures will differ widely. It should be noted that similar variation in gas/liquid ratios can also result from partial leakage of inclusion contents, as is also common in halite. The insert photograph shows a long, tubular, possibly primary inclusion in the same sample, at the same scale, in the process of necking down to form three smaller inclusions, only one having the bubble. Sample ER 64-112, Salar Grande, Chile, provided courtesy of George Erickson, U.S. Geological Survey. Fig. 34a,b. Brine inclusions (seminegative crystal shape; B) between anhydrite crystals (A) embedded in single crystal halite (H) from Rayburn dome, Louisiana. Several such inclusions were all parallel, suggesting the direction of stretching or shearing of host salt. From Roedder and Belkin (1979b).
Hence I conclude that the small vapor bubbles in many inclusions in salt (if not from leakage) represent mainly shrinkage on cooling from the in situ temperature. Roedder and Belkin (1979a) reported that 230 out of 317 liquid/vapor inclusions in bedded salt from the Waste Isolation Pilot Plant (WIPP) site in the Carlsbad area, New Mexico, homogenized between 20 and 50°C, and most of the remainder homogenized below 100°C. All Th values above 80°C were believed to be spurious (due to stretching during the experiment), and some never did homogenize - they continued to stretch during heating and hence maintained a vapor bubble even at >300°C. The rate of pressure increase with increasing temperature is small, as long as a vapor phase is present (Roedder and Bodnar, 1980, their Fig. 4), but once homogenization (Th) is reached, the rate jumps to ~12 bars/°C (Potter, 1977). If a piece of salt containing inclusions is heated rapidly, this pressure rise will cause decrepitation, even as low as 60°C (Roedder and Belkin, 1979a). If it is heated slowly, it will not decrepitate, but will merely expand or stretch by plastic deformation, even to temperatures as high as 250°C (Fig. 38). From the above it is obvious that inclusions in salt do not constitute a good geothermometer. Inclusions in nonplastic minerals such as anhydrite formed with the salt may, however, provide good thermometric data (e.g., Fig. 39; see also Petrichenko, 1973). The use of fluid inclusions in salt as geobarometers rather than geothermometers has a little more validity. The pressure in a gas inclusion at room temperature provides a minimum value for the pressure of formation. The actual pressure must have been larger as a result of the higher temperatures during formation, and any stretching of the host salt upon exposure to 1 atm surface pressure would also decrease the pressure remaining in the inclusions. One might ask why high pressure inclusions are ever found in salt if stretching is so rapid and effective. The explanation may lie in a combination of two aspects. First, the highest pressure gas inclusions seem to be water-free, and water helps greatly to increase salt plasticity. Second, the highest pressure inclusions are all very small. Recent studies on laboratory stretching of inclusions in fluorite from internal pressure (Bodnar and Bethke, 1984) have shown a strong positive correlation of stretching with inclusion size.
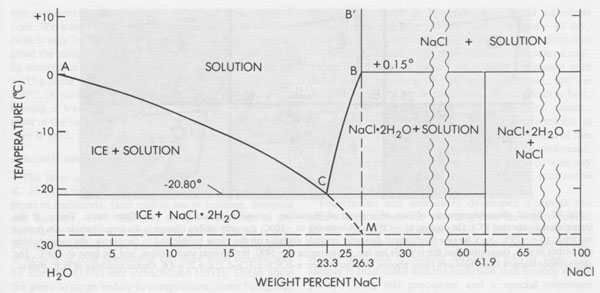
Low temperature microthermometry Although inclusions in salt may deform, they apparently seldom leak, and hence compositional data on the fluids, obtained by conventional low temperature microthermometric techniques (Hollister and Crawford, 1981), can be valid and useful. Unfortunately, of all fluid inclusions, those in salt are among the most difficult, experimentally. This is true both in the data collection and the data interpretation. The major problem is metastability in various forms. The simplest example involves an inclusion of NaCl-H2O brine in halite (Fig. 40). On cooling to +0.15°C, the fluid (B) in the inclusion should react with the solid walls to form the incongruently-melting compound hydrohalite, NaCl • 2H2O. This phase is very sluggish to nucleate and grow. Hence the inclusion fluid continues down the metastable line B-M. Normally nothing will happen to this metastable, supercooled, fluid until ~-70° or -80°C, when it may suddenly crystallize to a metastable solid assemblage of NaCl and ice. On warming, this will start melting at the metastable eutectic M at ~-28°C. If the temperature is held between -28°C and -20.8°C, the metastable liquid will eventually nucleate hydrohalite and cause the inclusion to change from a metastable mixture of liquid M and NaCl crystals to a solid mass of stable crystals of ice plus hydrohalite. The reaction with the walls will corrode them, making the inclusion visibly larger (and optically obscure). On heating, this hydrohalite should melt incongruently at +0.15°C to form liquid B and solid salt, but hydrohalite is not only reluctant to nucleate, it is reluctant to melt (Adams and Gibson, 1930) and sometimes crystals of it can be held at as much as 4°C over their melting point for more than an hour. So even on gradual heating, the last of the hydrohalite crystals usually decomposes several degrees above 0°C.
Fig. 37. Sequence of photographs of inclusion in salt crystal from Delaware basin, New Mexico, under uniaxial compression in crushing stage, applied perpendicular to plane of photographs, at 25°C. (a) Inclusion as found, before application of pressure. (b) Ten minutes after application of stress; the walls of the inclusion are becoming rough. (c) Eighteen minutes after application of stress; major changes in the walls have occurred, and the total volume of the inclusion cavity has decreased, eliminating the vapor bubble. From Roedder and Belkin (1977).
Fig. 38. Stretching of halite surrounding inclusions from internal pressure developed by heating. (a) Group of small primary fluid inclusions in primary chevron bedded salt from Delaware basin, New Mexico, as found. Several of the larger inclusions have very small vapor bubbles (arrows) that homogenize at ~49°C, and most inclusions have no bubbles. (b) Similar sample photographed at room temperature, after heating to 250°C over ~8 hours, holding at 250°C for 79 hours, and cooling to room temperature over ~10 hours. All inclusions, even very small ones, now have a vapor bubble, and homogenize at 180 to 273°C indicating deformation of the host crystal. From Roedder and Belkin (1978). Fig. 39. Fluid inclusion (presumably primary) containing liquid (L) and vapor (V) in anhydrite crystal (A) from Oakwood salt dome, East Texas. From Dix and Jackson (1982).
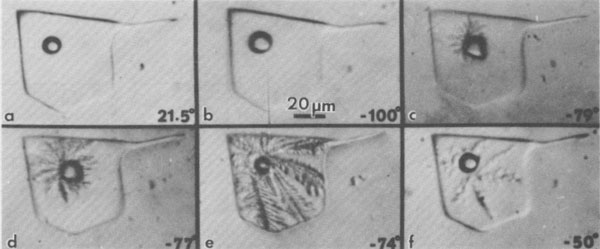
The behavior of multicomponent brines, with significant Ca and/or Mg, is recognizably different from the above, but plagued with even more serious problems from metastability. Such inclusions may be extremely difficult to freeze. Most form rigid glasses on cooling (Angell and Sare, 1970) that are actually metastable supercooled liquids. Glasses of this type will not crystallize at all at low temperatures (-180°C); only on holding in the optimum temperature range for nucleation and crystallization can even a rough estimate of the eutectic temperature be made in those few inclusions that form any solids at all (Fig. 41). Once they have crystallized, the melting on warming can be used, along with available phase equilibrium data (e.g., Crawford, 1981), to make some estimates of composition. Some fluid inclusions in salt show eutectic (metastable) melting at ~-28°C; this behavior indicates essentially pure NaCl-H2O inclusions, i.e., fresh water plus salt. Others show eutectic melting at ~-35°C, suggestive of MgCl2-NaCl mixtures. However, most inclusions, from both bedded and dome salt, show eutectic melting at -50°C or lower; these almost certainly require the presence of significant CaCl2.6 (The addition of KCl to these mixtures causes relatively little additional drop in the eutectic temperature.) As discussed later, the inclusion type of most significance to nuclear waste disposal, the pure NaCl-H2O inclusion, is also the easiest of these types to recognize by the freezing procedure. Amount and nature of H2O present Bedded salt may contain several percent total H2O (Roedder and Belkin, 1979a), but some dome salts have only ~30 ppm H2O (Knauth and Kumar, 1981). It is important to know both the amounts and kinds of H2O present in a salt bed for the engineering design of a waste storage site, since these various kinds will behave differently under the thermal pulse from the waste. Although water is present in three major forms (hydrous minerals, intragranular inclusions, and intergranular inclusions), in the past many "water analyses" have been made by the simple procedure of heating and weighing, and the results were then used in engineering design. Roedder and Bassett (1981) showed that many if not most of the published analyses of water in salt contain serious and in part systematic errors and are generally low. These errors are caused by various combinations of extreme sample heterogeneity, multiple sources of H2O, strong sample bias from sample selection and preparation procedures, and inadequate analytical techniques. In particular, many of the published DTA and TGA data on the dehydration of hydrous saline minerals are mutually contradictory and need a thorough restudy.
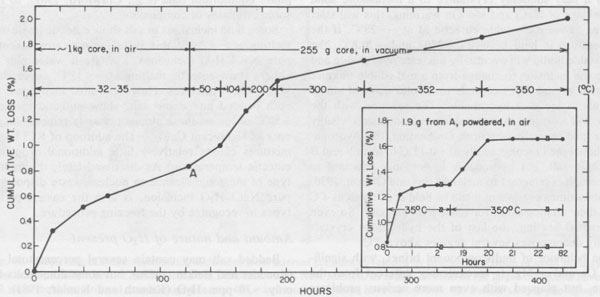
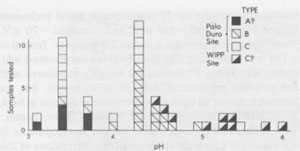
Figure 42 provides an example of one of the types of rate problems involved in water analysis of a 1-kg piece of core. We wanted to avoid losing the intergranular fluids (which may be 50% of the total), so we did not crush or grind the sample. After 122 hours at 32-35°C in air, the 1kg segment of core was still losing weight, so we broke off a 255-g piece which was heated up to 300°C and finally to 350°C, in vacuum, and even after 301 additional hours of heating, it was still losing weight. No panacea exists, but most of the sources of error can be minimized if adequate care is used, particularly in sample handling. Amounts and nature of gases present The laser-activated Raman spectrometer (Rosasco et al, 1975) is almost perfectly suited for the analysis of gases in inclusions, (and sulfate ion in solution, Rosasco and Roedder, 1979; Dubessy et al., 1980), but to my knowledge has yet to be applied to gas inclusions in salt. Numerous studies of the gases evolved during dissolution or heating of bulk samples of salt have been summarized by Roedder (1972) and Petrichenko (1973). These show the gases to range widely in composition, some being high in CO2, others high in CH4, and still others high in C2H6, N2, etc. N2 can constitute <=95% (Vil'denberg et al., 1978). The N2 and other gases may stem, in part, from the decomposition of algal mats such as are seen in some modern day saline environments. Norman and Bernhardt (1984) reported also CS2 and H2S, by mass spectrometry of the gases evolved on decrepitation. The pressure, composition, and amount of gases are all pertinent to waste disposal, since any tendency toward popping salt behavior will certainly be aggravated by a thermal pulse. Entirely apart from the practical problems, the origin(s) of these gases is an interesting and essentially untouched geochemical problem in itself. In addition to the bulk constituents, the minor and trace gases may be of interest. Any fluid in contact with the atmosphere will eventually equilibrate with it in respect to the noble gases. Although the solubilities of the noble gases in brines are lower than in fresh water (Smith and Kennedy, 1983), carefully selected fluid inclusions in salt may provide the best available samples for determination of the elemental and isotopic ratios of the noble gases in early atmospheres (Roedder, 1984). Eh and pH determinations on inclusion fluids Petrichenko and Shaydetskaya (1968; summarized in Petrichenko, 1973) were the first and only workers to measure the Eh of any fluid inclusion. They inserted a pair of electrodes (platinum and calomel, using Zobell's procedure) through 0.8- to 1.0-mm holes drilled into very large fluid inclusions in water-clear recrystallized halite from the Artemousk rock salt deposit in the Donbass, USSR. Atmospheric air was excluded, and a correction of +245 mV was made, "following Garrels" (no reference given). In other experiments, the inclusion fluid was pulled into a capillary containing the miniature electrodes. The results on 27 single-phase liquid inclusions ranged from -10 to -130 mV, and averaged -70 mV. Two-phase inclusions gave higher Eh readings, from 0 to + 150 mV, indicating that their bubbles represented infiltrating air. These Eh values are roughly consistent with the possible presence of CH4 in these brines, but the interpretation of all such measurements is difficult at best, even with much larger sample volumes (pers. comm., D.C. Thorstenson, U.S.G.S.). Recent developments in subminiature Eh electrodes may permit extensions of this work to the smaller, more common sizes of inclusions, but all such determinations should be compared with any available mineralogical evidence as to the Eh, such as the presence of CH4, sulfides, etc. Petrichenko and coworkers developed a simple but effective procedure for exposing fluid inclusions in salt for Eh, pH, and chemical analysis. A tiny stream of water is run over the surface of the sample (under the microscope) until the remaining inclusion wall is thin. The sample is then dried and the inclusion wall broken with a probe. Using this procedure and a special miniature platinum electrode, Petrichenko and coworkers measured the pH of inclusions as small as 0.5 mm from a series of salt deposits in the USSR. Glass electrodes were used on very large inclusions, and small inclusions were studied using the colorimetric method of Kalyuzhnyi (1957, 1960). Most of their determinations fell in the range pH = 3.5 to 6.4, as might be expected for alkaline-earth chloride solutions. We have used a modification of Kalyuzhnyi's procedure to determine the pH of inclusions in recrystallized bedded salt from the Palo Duro basin, opened using the Petrichenko water-thinning technique. Instead of the special organic films, prepared from the lining of a chicken egg (pers. comm., Vl. A. Kalyuzhnyi, IGiGGI, L'vov, USSR, 1970), we used commercially available narrow-range pH test papers, each covering a total pH range of l.6 to l.8 pH units. Once the inclusions were cracked open, a tapered point of the appropriate pH test paper was touched to the liquid and the color change matched under the binocular microscope with the color test strip provided by the manufacturer. The color changes under these conditions of sample volume, lighting, and observation were verified using commercial pH buffer solutions. Many of the determinations were verified by the use of pH papers with wider ranges, and/or with adjacent, overlapping, narrow-range papers. One inclusion was opened and tested three times: immediately, after 20 mins., and after 60 mins. exposure of the opened inclusion to air, and no change was noted. Any given pH determination is subject to an uncertainty of visual match of ~±0.3 pH unit, so a conservative estimate of uncertainty on the values obtained would be ±0.5 pH units. The results of 40 determinations on Palo Duro salt, and 10 on WIPP salt are given in Figure 43, categorized in terms of preliminary textural examinations by S. Hovorka of the University of Texas at Austin (pers. comm.). Some of the pH differences found correlate with textural type or locality, but more work is needed before any conclusions may be reached in this regard.
Fig. 43. Results of pH determinations on primary fluid inclusions (?0.5 mm) in recrystallized bedded salt from Palo Duro basin, Texas and the WIPP site, Carlsbad, New Mexico. The three types of environment in which recrystallization of the host salt took place are based on work of S. Hovorka (pers. comm., 1983). Type (A) is salt from just below a sedimentary facies change, and may represent material that was dissolved and recrystallized by fluids from this later stage. Type (B) is coarse clear salt filling crosscutting solution cavities within the salt beds. Type (C) is coarse clear salt from recrystallization within the salt beds. The samples from the WIPP site would probably be categorized as type C.
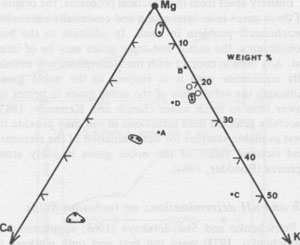
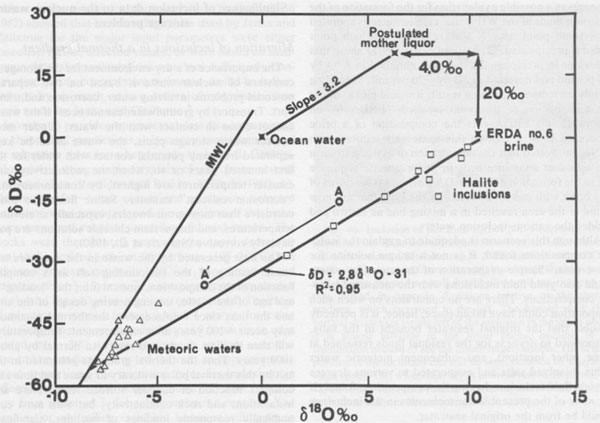
Solutes present in aqueous fluid inclusions Centimeter-sized inclusions can be easily opened and analyzed, but are so rare in occurrence that they are not very useful in tracing geological processes. The much more common inclusions in the l-mm range have been studied by a variety of quantitative, semiquantitative, and qualitative methods, particularly in the USSR (summarized in Petrichenko, 1973). Quantitative analysis of the smaller inclusions requires very sensitive micro or ultramicro methods. Holser (1963, 1968) pioneered in this field, but relatively little additional work has been reported since then. At the U.S. Geological Survey we have recently been extracting and analyzing single inclusions, mostly >=500 µm on an edge (i.e., >=~1.6 x 10-4 g), using inductively coupled plasma spectroscopy and other methods. The data are far from complete and full details will be presented elsewhere, but a few of the preliminary results can be given here. Figure 44 is a plot of the relative amounts of Ca, Mg, and K found in analyses of "large" inclusions (>= 10-4 g) from recrystallized bedded salt from the Palo Duro basin and two from Lyons, Kansas. (Unfortunately, these are not the same inclusions on which pH was determined.) By integrating these data with sample classification based on textural studies of these cores by S. Hovorka at the University of Texas at Austin (pers. comm.) it is safe to conclude that different fluids were present at different times in these salt beds. This conclusion is also supported by the fact that inclusions separated by less than one millimeter can have widely disparate compositions, far greater than the analytical uncertainty, as evidenced by the results on duplicate samples (Fig. 44). Sulfate is low, particularly in the high-Ca fluids, and Br, determined with the Dionix Model 14 ion chromatograph, ranges from <80 to ~5000 ppm. Thus some of these fluids may be original residual bitterns left from crystallization of halite from seawater, some may be from halite recrystallization, some may be from dissolution of K-Mg beds, and a variety of rock-water interactions such as dolomitization may have been involved. At this stage we cannot determine the sequence of these fluids, nor can we safely assign any to saline depositional basin or later diagenetic fluids, but eventually such assignments may be possible. Isotopic signatures of H and O in inclusion fluids The isotopic signatures of H and O in the inclusion fluids (normally stated in terms of δD and δ18O relative to SMOW, Standard Mean Ocean Water) provide information on the possible sources and processes involved in the history of the fluids present in inclusions. The interpretation of such isotopic data, as with other geochemical variables, does not offer a unique solution, but does provide useful additional constraints on possible models for the derivation of the fluids. The results of a study of 9 "large" inclusions in recrystallized bedded salt from the WIPP site are summarized in Figure 45.
The fluids evaporating in a saline basin to yield salt beds are normally thought of as essentially seawater residues, but the available δD and δ18O data on fluid inclusions in salt show that this generalization is incorrect. As an example, the 9 analyses of water from fluid inclusions from the WIPP site plot to the lower right of Standard Mean Ocean Water (SMOW) on Figure 45. How can such fluid inclusion waters be obtained from seawater? Many processes can cause fluids to move on such a plot. Mixing of two waters will yield intermediates along a straight mixing line between the end members. Analyses by J.R. O'Neil, U.S. Geological Survey, of formation waters from various wells in the WIPP area (Lambert, 1978) show very low δD and δ18O, and plot in the lower left of Figure 45, near the meteoric water line. Mixtures of such fluids with seawater would lie between these two points. Partial evaporation of seawater will leave a residual fluid enriched in the heavy isotopes of both H and O, causing it to move toward the upper right, along a line whose slope is controlled by the temperature, humidity, and kinetic factors (Sofer and Gat, 1975). The oxygen in most sediments is enriched in 18O relative to most waters, and hence exchange between water and rock (particularly with clastic carbonates and clays) will cause the water to shift to the right (i.e., it will show an "oxygen shift"). Any H- or O-bearing phase that precipitates from a fluid will generally have different isotopic values for H and O than the fluid, and such precipitation will drive the remaining fluid along a line directly away from the plotted isotopic composition point for the solid. Thus Frape and Fritz (1982) showed that low-temperature hydration of rock silicates, forming clays, moved the residual water toward the upper left. Similarly, reaction of a fluid with such minerals (including organic matter), or mixing with fluid from their dehydration, will move the original fluid toward them along a mixing line. Such reactions may be the explanation for the unusual hydrogen composition (δD -69.2 per mil, along with δ18 -5.4 per mil) found for a fluid inclusion in halite from the Oakwood dome, Texas (Kreitler and Dutton, 1983). J.R. O'Neil (as reported by Schneider and Trask, 1983, p. 33, and pers. comm.) has suggested the following scenario as a possible explanation for the formation of the inclusion fluids at the WIPP site: (1) Seawater evaporated until some point near X was reached, at which point gypsum precipitated. (2) Data of Sofer (1978) show that the water in precipitated gypsum is enriched in δ18O by ~4 per mil and depleted in δD by ~20 per mil, depending mainly on temperature; as a result, it would plot at about the composition of the point labelled "ERDA 6" on Figure 45. (3) ERDA 6 is the composition of a brine recovered from a well in this same salt sequence, so O'Neil suggested that the dehydration of gypsum to form the abundant anhydrite beds in this evaporite sequence led to the formation of the ERDA 6 brine. (4) Mixtures of this brine with meteoric waters of the type that are now found in the area resulted in a mixing line as shown and yielded the various inclusion waters. Although this scenario is adequate to explain the isotopic compositions found, it is not a unique solution for these data.7 Simple evaporation of the meteoric waters could also yield fluid inclusions with the measured isotopic compositions. There are no constraints on when such evaporation could have taken place; hence, it is perfectly feasible that the original seawater brought in the salts, evaporated to dryness (or the residual fluids remained at some other location), and subsequent meteoric water influx dissolved salts and evaporated to various degrees to yield fluid inclusions having the compositions found. If so, none of the present water molecules in the inclusions would be from the original seawater. L. Paul Knauth and Mark Beeunas of Arizona State University and I have been making a similar study of inclusions from the Palo Duro basin. Some of the early isotopic results they got on some of my samples were strange and difficult to explain. They then found that the extraction procedure they had used in their vacuum line on my samples could introduce errors much larger than the instrumental error (see A and A' in Fig. 45; these are two analyses of a single sample. A is believed to represent the correct values), and subsequent studies (Beeunas and Knauth, 1983) yielded much more consistent and interpretable data that plot near to those shown on Figure 45. Their model involves various degrees of evaporation of a mixture of seawater and local meteoric waters, though the amount of seawater involved must be small unless an extremely light composition is assumed for the meteoric water component. B.F. Jones (U.S.G.S.) has pointed out (pers. comm., 1984) that in addition to the changes from evaporation, hydrate precipitation, and other mineral reactions, the wide variety of possible sources for meteoric fluids to mix with solutes from the salt beds make a unique interpretation of the isotopic data very difficult. These sources can include sheet floods, rain, dew, stream waters, etc. (e.g., Knauth et al., 1980, their Fig. 2).
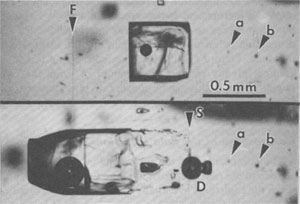
Significance of inclusion data to the nuclear waste storage problem Migration of inclusions in a thermal gradient The importance of a dry environment for the storage of canisters of nuclear waste is based on two separate potential problems involving water, corrosion and transport. Transport by groundwater cannot occur if the waste cannot come in contact with the water. Under most nuclear waste storage plans, the waste is to be kept separated from any potential contact with water for the first hundred years or so, when the radioactivity (and canister temperature) are highest, by containment in a "corrosion-resistant" canister. Saline fluids are more corrosive than most groundwaters, especially at elevated temperatures, and magnesium chloride solutions are particularly corrosive (Stewart et al., 1980). The heat generated by the waste in the canisters will cause heating of the surrounding salt as a complex function of the composition, amount (i.e., the "loading"), and age of the waste, the engineering design of the site, and the time since emplacement; the thermal maximum may occur ~100 years after emplacement. Temperatures will then decline slowly and return to normal by about 1000 years. Thus the thermal gradients generated in the salt by this thermal pulse will vary in space and time as a complex function of canister surface temperature and installation, and rock conductivity, but with most economically reasonable loadings of nuclides, significant volumes of salt surrounding a canister may be subjected to gradients of ~ l.5°C/cm for periods of years. What happens to a fluid inclusion in salt in such a thermal gradient? Although the thermal coefficient of NaCl solubility in water is small in the range of ambient temperatures expected, it has a finite positive value, so salt will dissolve on the warmer side of the inclusion and precipitate on the cooler side, causing the inclusion to move up the thermal gradient, toward the heat source, exactly where fluids are not wanted.8 The amount of brine that might reach a canister in this way, and its capacity to affect cannister performance, must be considered in the design of any proposed nuclear waste repository in salt. The theory of the migration of fluid inclusions in KCl and NaCl in thermal (and gravitational) fields has been examined in some detail, and a rather extensive literature exists. Jenks and Claiborne (1981) summarized this literature and made calculations, which they claimed to be "reasonably conservative," of the rates and total volumes of brine expected to migrate into a given emplacement hole in bedded salt over the first 100 years. These calculations showed that inclusion migration was of relatively minor concern in the engineering design of a nuclear waste repository. However, Roedder and Chou (1982) showed that because the values used by Jenks and Claiborne for the major input parameters were either nonconservative, selected values, or were based on inadequate data, their calculations were not sufficiently conservative. Truly conservative estimates should be larger, perhaps by as much as two orders of magnitude, than those made by Jenks and Claiborne.9 In order to provide experimental data to compare with the theoretical predictions, laboratory measurements of the migration rates have been made at the U.S. Geological Survey, using single crystals of salt from several proposed sites (Roedder and Belkin, 1979a,b; 1980a,b). Blocks of salt ~1 cm square and containing selected inclusions were prepared, and the positions of the inclusions measured relative to scribed fiducial marks. The blocks were then heated gradually (to avoid decrepitation) to the desired ambient temperature (108-260°C) and a thermal gradient, generally l.5°C/cm, superimposed and maintained for 3-10 days. The samples were then cooled slowly and the inclusion positions remeasured. The rates of movement ranged from 1.2 to 5.4 cm/yr. for cubic inclusions 1 mm on an edge; inclusions 0.1 mm on an edge moved only ~30% as fast. Increase in ambient temperature and/or gradient increased the rate, in approximately direct proportion. The migration rates for inclusions in different parts of a given sample, however, were found to vary by a factor of 3, for as yet unknown reasons. The three major controlling variables, however, still seem to be inclusion size, ambient temperature, and temperature gradient. Theoretical considerations and some experimental studies suggest that the migration rate may also be related to the fluid composition, the presence (and volume) of a gas bubble, the gas pressure in such a bubble, mechanical strain in the host salt, dislocation abundance and nature, crystallographic direction, and perhaps radiation damage and gravity. Thus radiation damage in NaCl, forming colloidal sodium, might affect the migration rates for fluid inclusions moving through it, as well as the pH of the fluid (Panno and Soo, 1983). Unless sodium or chlorine is actually lost to the system, however, changes in the chemical composition of the fluid could only be local effects. Some of the features of inclusion shape and motion have been analyzed theoretically and experimentally (e.g., Yagnik, 1983a), but several interesting and in part unexplained phenomena were noted in the present work (Fig. 46).10
Fig. 46. Inclusion in salt from ERDA No. 9 borehole, Waste Isolation Pilot Plant site, Carlsbad, New Mexico, before (above) and after (below) a 156-hour run at 202°C ambient and 1.5°C/cm gradient. The large inclusion has split into a large liquid-rich part with trailing fins and a small dumbbell-shaped gas-rich part (D) that moved in opposite directions relative to the thermal gradient, which increased to the left. The fiducial mark (F, a vertical scratch) is visible left of the inclusion in the upper photo; it is almost invisible in the lower photo, because of the illumination needed to see the (much larger) bubble. The original position of the inclusion can still be seen, outlined by a series of small specks (S), which also act as internal reference points, but various other reference points, such as (a) and (b), are visible in both photos. From Roedder and Belkin (1980a).
The above tests provide information only on the migration of fluid inclusions within single crystals. As most rock salt has a grain size of ~1 cm, under these conditions, most inclusions will reach a grain boundary within a year or so. What happens then? Small scale experimental studies (Roedder and Belkin, 1980a) show that on intersecting a grain boundary, most fluid inclusions do not cross, but instead leak their fluid into the interface. How the fluid will then behave involves too many variables to permit meaningful quantitative modelling at this time, and the interpretation of some larger scale laboratory experiments is ambiguous (Lambert, 1980; Roedder and Belkin, 1980a). Full scale, rather long term experiments at the selected site itself, carefully planned to avoid ambiguity in interpretation of the data obtained, seem to present the only likely solution. Bedded salt deposits have been subjected to the natural geothermal gradient for geologic time, but careful petrography of oriented samples suggests that there has been no downward movement of the inclusions during the intervening hundreds of millions of years. Why not? Possible explanations include the following: (1) The movement may have been too small to detect. (2) The driving force downward from the geothermal gradient may be about equal and opposite to the driving force upward from the gravitational field. (3) A threshold gradient may exist below which inclusions will not move. Explanation (l) seems unlikely since even 10 µm of movement should be detectable. Explanation (2) requires the balancing of two opposing factors and hence seems unlikely.11 Explanation (3) involves unknowns such as interface kinetics and dislocation behavior and hence can only be estimated at this time. Pigford (1982, 1983) argued that the threshold gradient greatly reduces the amount of brine that will migrate and that this can be conservatively estimated, but Chou (1983) took exception to several aspects of Pigford's calculations. Composition of the fluid inclusions The composition of the inclusions in salt is of consequence to the rate of corrosion of the waste canister, and, when the canister is eventually breached, to the corrosion of the waste form itself, but is also important in several other aspects of nuclear waste site selection. Insofar as the inclusion chemical and isotopic data provide information on the processes leading to the original formation of the deposit, they may help in selecting an optimum site for the repository. More important, perhaps, they may indicate the source of the fluids. If, as has sometimes been observed in dome salts (Roedder and Belkin, 1979b), the inclusions show eutectic (metastable) melting at ~-28°C, essentially fresh water and dissolved NaCl is involved; significant amounts of CaCl2 and/or MgCl2 will yield much lower eutectic melting. The difference in behavior of the two types is readily apparent under the microscope, so that even a single H2O-NaCl inclusion among many multi-ion inclusions is easily spotted. Nearly pure NaCl-H2O fluid in inclusions in a salt bed or dome can have three possible origins: (l) It can be sea water trapped in the first-formed salt crystals, before evaporation and crystallization caused much buildup of other ions. (2) It can be water evolved by the dehydration of clay minerals or the conversion of other phases such as gypsum to anhydrite during diagenesis. (3) It can be fresh water which has penetrated the salt at some unknown time and has been trapped (Stewart et al., 1980). The third possible origin may have potential significance to the possible use of these domes for nuclear waste storage. At the depth from which the originally bedded salts have flowed to form these domes (commonly ~10-12 km), the fluids in the pores of the surrounding sediments were almost certainly highly saline formation waters, containing significant ions other than Na and Cl. However, during its rise, the salt dome must have penetrated those aquifers which now surround it and contain essentially fresh water. If fracturing occurred during this rise, or subsequently (and we have no knowledge of the age of these inclusions), fresh or brackish waters could enter the salt and become trapped as saturated brine. The corollary is that if this happened in the past, it might also happen in the future. Knauth and Kumar (1983) showed from isotopic studies that unlike the primarily formation water encountered in other salt domes, the water in current brine leaks at the Avery Island salt dome mine in Louisiana was essentially meteoric water. They indicate that these leaks may be related to the shallow depth of the Avery mine, close proximity of the salt top to the surface, absence of caprock, and/or duration and technique of mining. Whether such leaks could be part of a possible scenario leading to significant transport of nuclides beyond the dome remains to be demonstrated.
Conclusions Consideration of salt deposits for possible storage sites of nuclear waste has led to renewed interest in the natural fluids in salt. Most salt contains at least some H2O as hydrous minerals and as fluid inclusions, either intracrystalline or intercrystalline; it may also contain oil or CO2, CH4, or other gases. Primary intracrystalline fluid inclusions in primary bedded salt are usually very small, but ordinary petrographic studies of them can provide valuable information on the environment of the original salt basin. Later and larger primary inclusions in recrystallized salt provide information on the fluids present after the original salt crystallization, but the specific time of this recrystallization is generally unknown; it could have been at any time following crystallization on the basin floor. The original textures and inclusions in bedded salts are largely lost during the deformation that forms salt anticlines and domes. The origin of the few fluid inclusions found in domal salt is usually ambiguous. High pressure gas inclusions containing CO2, CH4, N2, or other gases, of generally unknown source, are common in dome salt locally and may be in high enough concentration to cause hazards during mining from sudden large-size blowouts. Geothermometry by the homogenization method on fluid inclusions in halite is essentially hopeless, as a result of the plasticity of halite, but the gas inclusions provide a possibly useful minimum pressure of formation. Low temperature microthermometry provides evidence that the brines in many fluid inclusions, both in bedded and dome salt, are high in Ca and/or Mg. Larger fluid inclusions have been extracted for determination of pH (moderately acid; ~3 to 6), Eh (slightly reducing; -10 to -130 mv), and chemical analysis. Most are high density chloride solutions (~l.3 g/cm3) of Na, K, Ca, and Mg, but in widely differing ratios, sometimes even in adjacent inclusions, apparently as a result of different fluid sources at different times. Sulfate is low, particularly in the high-Ca fluids, and Br ranges from <80 to at least 5000 ppm. Isotopic studies of the H and O in water from inclusions place constraints on the processes yielding the fluids, but are ambiguous in interpretation. Migration of fluid inclusions up the thermal gradient established by a radioactive waste canister will tend to concentrate at the canister the fluids from inclusions from some distance around. Estimates of the rates and total volumes of fluid that must be dealt with in a repository design are subject to rather large uncertainties. In summary, the fluids in salt are both advantageous and deleterious. They are advantageous in that they tell us much about the environment and subsequent history of the salt deposit; as such, they also suggest what might happen in the future at a nuclear waste site in salt. They are deleterious in that they introduce some problems in the engineering design of a nuclear waste installation that must be separately evaluated at each suggested site. Acknowledgments I have been helped by discussions with many individuals, particularly at the Bureau of Economic Geology of the University of Texas at Austin, during the work leading to this paper. I am most particularly indebted to the following who coauthored a series of papers and unpublished manuscripts with me, or provided unpublished material, on which sections of this paper are based: H.E. Belkin, J.R. O'Neil, C.M. Johnson, L.D. White, E.L. Libelo, A.F. Dorrzapf, Jr. and W.M. dAngelo of the U.S. Geological Survey, and R. Bassett (formerly of the University of Texas at Austin). The manuscript profited from reviews by D.E. White, B.F. Jones, H.E. Belkin and E. H. Roseboom, Jr. (USGS), W. Harrison (ANL), and S. Hovorka, C.W. Kreitler, and R.S. Fisher (Univ. Texas). Partial funding for this work was provided by DOE Contract No. DE-AC97-83WM46651.
References Adams, L.H. and Gibson, R.E. (1930) The melting curve of sodium chloride dihydrate. An experimental study of an incongruent melting at pressures up to twelve thousand atmospheres. Journal of the American Chemical Society, 52, 42524264. Angell, C.A. and Sare, E.J. (1970) Glass-forming composition regions and glass transition temperatures for aqueous electrolyte solutions. Journal of Chemical Physics, 52, 1058-1068. Arthurton, R.S. (1973) Experimentally produced halite compared with Triassic layered halite-rock from Cheshire, England. Sedimentology 20, 145-160. Baar, C.A. (1977) Applied Salt-Rock Mechanics l. Elsevier Scientific Publishing Company, Amsterdam 294 p. Baes, C.F., Jr., Gilpatrick, L.O., Kitts, F.G., Bronstein, H.R., and Shor, A.J. (1983) The effect of water in salt repositories. Final Report. Oak Ridge National Laboratory ORNL-5950, 62 p. Beeunas, M.A. and Knauth, L.P. (1983) Isotopic composition of fluid inclusions in Permian halite-implications for the isotopic history of sea water. (abstr.) Geological Society of America Abstracts with Programs, 15, 524. Belchic, H.C. (1961) The Winnfield salt dome, Winn Parish, Louisiana. In Interior Salt Domes and Tertiary Stratigraphy of North Louisiana, 1960 Spring Field Trip Guide Book, p. 29-47. Shreveport Geological Society, Shreveport, LA. Bodnar, R.J. and Bethke, P.M. (1984) Systematics of stretching of fluid inclusions I: Fluorite and sphalerite at I atmosphere confining pressure. Economic Geology, 79. 141-161. Bradley, W.H. (1965) Vertical density currents. Science 150, 1423-1428. Chou, I-Ming (1983) Remarks on "Migration of brine inclusions in salt". Nuclear Technology, 63, 507-508. Commission to investigate Wapno salt mine flooding (1977) Report to Pila Voivodship Geological Office, Poland, 9 p. (in Polish). Crawford, M.L. (1981) Phase equilibria in aqueous fluid inclusions. Mineralogical Association of Canada Short Course Handbook, 6, 75-100. Dean, W.E. (1978) Trace and minor elements in evaporites. In W.E. Dean and B.C. Schreiber, Eds., Marine Evaporites. p. 86-104. Society of Economic Paleontologists and Mineralogists, Short Course No. 4. Dellwig, L.F. (1955) Origin of the Salina salt of Michigan. Journal of Sedimentary Petrology, 25, 83-110. Dix, O.R. and Jackson, M.P.A. (1982) Lithology, microstructures, fluid inclusions, and geochemistry of rock salt and of the cap-rock contact in Oakwood dome, East Texas: Significance for nuclear waste storage. Bureau of Economic Geology, The University of Texas at Austin, Report of Investigations No. 120, 59 p. Dubessy, Jean, Geisler, Dominique, Kosztolanyi, Charles, and Vernet, Michel, (1980) Determination by Raman spectroscopy (M.O.L.E. microprobe) of the sulphate ion in the fluid inclusions of recent halites (Camargue, France). Science de la Terre, 24, 197-212 (in French, English abstract). Frape, S.K. and Fritz, P. (1982) The chemistry and isotopic composition of saline ground waters from the Sudbury Basin, Ontario. Canadian Journal of Earth Sciences, 19, 645-661. Friedman, G.M. (1978) Depositional environments of evaporite deposits. In W.E. Dean and B.C. Schreiber, Eds.. Marine Evaporates, p. 177-188. Society of Exploration Paleontologists and Mineralogists Short Course No. 4. Groat, C.G. (1981) Rig lost; lake fills salt mine. Geotimes, 26, (3), 14. Hanford, C.R. (1981) Coastal sabkha and salt pan deposition of the Lower Clear Fork Formation (Permian), Texas. Journal of Sedimentary Petrology, 51, 761-778. Hollister, L.S. and Crawford, M.L., Eds. (1981) Fluid Inclusions: Applications to Petrology. Mineralogical Association of Canada Short Course Handbook, 6, 304 p. Holser, W.T. (1963) Chemistry of brine inclusions in Permian salt from Hutchinson, Kansas. In A.C. Bersticker, Ed., Symposium on Salt. p. 86-95. Northern Ohio Geol. Soc., Cleveland, Ohio. Holser, W.T. (1968) Chemistry of brine inclusions in Permian salt from Hutchinson, Kansas. (abst.) Geological Society of America Special Paper 88, 537. Holser, W.T. (1979) Mineralogy of evaporites. In R.G. Burns, Ed. Marine Minerals, p. 211-294. Mineralogical Society of America Short Course Notes. Vol. 6, Washington, D.C. Hoy, R.B., Foose, R.M., and O'Neill, B.J., Jr. (1962) Structure of Winnfield salt dome, Winn Parish, Louisiana: American Association of Petroleum Geologists Bulletin, 46, 1444-1459 (see also Geological Society of America Special Paper 88, p. 409-410, 1968). Jenks, G.H. and Claiborne, H.C. (1981) Brine migration in salt and its implications in the geologic disposal of nuclear waste. Oak Ridge National Laboratory, ORNL 5818, 164 p. Kalyuzhnyi, V.A. (1957) Results on pH measurements in solutions from liquid inclusions. Geokhimiya, 1957, (l), 77-79 (in Russian; transl. in Geochemistry (l) 93-96, 1957). Kalyuzhnyi, V.A. (1960) Methods of study of multiphase inclusions in minerals. Izdatel. Akad. Nauk Ukrainskoy RSR, Kiev, 169 p. (in Ukrainian). Knauth, L.P. and Kumar, M.B. (1981) Trace water content of salt in Louisiana salt domes. Science, 213, 1005-1007. Knauth, L.P. and Kumar, M.B. (1983) Isotopic character and origin of brine leaks in the Avery Island salt mine, south Louisiana, U.S.A. Journal of Hydrology, 66, 343-350. Knauth, L.P., Kumar M.B. and Martinez, J.D. (1980) Isotope geochemistry of water in Gulf Coast salt domes. Journal of Geophysical Research, 85, 4863-4871. Kreitler, C.W. and Dutton, S.P. (1983) Origin and diagenesis of cap rock, Gyp Hill and Oakwood salt domes, Texas, Bureau of Economic Geology, The University of Texas at Austin, Report of Investigations No. 131, 58 p. Lambert, S.J. (1978) Geochemistry of Delaware Basin groundwaters. New Mexico Bureau Mines and Mineral Resources, Circular 159, 32-38. Lambert, S.J. (1980) Mineralogical aspects of fluid migration in "Salt Block 11" experiment. Sandia National Laboratories, Sand 79-2423, 24 p. Linke, W.F. (1965) Solubilities, Inorganic and Metal-Organic Compounds, Vol. II, 4th Edn. American Chemical Society, Washington, D.C., 1914 p. National Academy of Sciences-National Research Council (1957) The disposal of radioactive waste on land, Report of the Committee on Waste Disposal, H.H. Hess, Chairman. National Academy of Sciences, Washington, DC, Pub. 519, 142 p. National Academy of Sciences-National Research Council (1966) Report of Committee on geologic aspects of radioactive waste disposal, J.E. Galley, Chairman. Report to the Division of Reactor Development and Technology, United States Atomic Energy Commission, 99 p. National Academy of Sciences-National Research Council (1970) Disposal of solid radioactive wastes in bedded salt deposits, Report by the Committee on Radioactive Waste Management, J.C. Frye, Chairman. National Academy of Sciences-National Research Council, Washington, DC, 28 p. National Academy of Sciences (1983) A study of the isolation system for geologic disposal of radioactive waste. Waste Isolation Systems Panel, Board on Radioactive Waste Management, Commission on Physical Sciences, National Research Council, 345 p. Norman, D.I. and Bernhardt, C.B. (1984) Analysis of gases in inclusions from evaporites, Salado Formation, New Mexico. Geochimica et Cosmochimica Acta (in press). Nurmi, R.D. and Friedman, G.M. (1977) Sedimentology and depositional environments of basin-center evaporites, Lower Salina Group (Upper Silurian), Michigan Basin. In J.H. Fischer, Ed. Reefs and Evaporites-Concepts and Depositional Models, p. 23-52. American Association of Petroleum Geologists, Studies in Geology No. 5. Panno, S.V. and Soo, P. (1983) An evaluation of chemical conditions caused by gamma irradiation of natural rock salt. Brookhaven National Laboratory Report NUREG-33658. (Submitted for publication in Nuclear Technology, 1984). Petrichenko, O.I. (1973) Methods of study of incusions in minerals of saline deposits. "Naukova Dumka" Pub. House, Kiev (in Ukrainian; transl. in Fluid Inclusion Research-Proceedings of COFFI, 12, 214-274, 1979). Petrichenko, O.I. (1977) Atlas of Microinclusions in the Minerals of Saline Rocks. Izdatelstvo Naukova Dumka, Kiev, 182 p. (in Russian). Petrichenko, O.I. and Shaydetskaya, V.A. (1968) Physicochemical conditions of recrystallization of halite in rock salts. In Mineralogical Thermometry and Barometry, vol. I, p. 348351. "Nauka" Press, Moscow (in Russian). Pigford, T.H. (1982) Migration of brine inclusions in salt. Nuclear Technology, 56, 93-101. Pigford T.H. (1983) Reply to "Remarks on `Migration of brine inclusions in salt' ". Nuclear Technology, 63, 509-510. Pigford, T.H. and Choi, J.-S. (1976) Effect of fuel cycle alternatives on nuclear waste management. In R.G. Post, Ed. Proceedings of the Symposium on Waste Mangement, Tucson, Arizona, p. 39-57. ERDA CONF-761020. Pines, David, ed. (1978) [Nuclear fuel cycles and waste management] Reviews of Modern Physics (Supplement), 50, part II, 185 p. Potter, R.W., II (1977) Pressure corrections for fluid-inclusion homogenization temperatures based on the volumetric properties of the system NaCl-H2O. Journal of Research of the United States Geological Survey 5, 603-607. Potter, R.W., II, Clynne, M.A. and Brown, D.L. (1978) Freezing point depression of aqueous sodium chloride solutions. Economic Geology, 73, 284-285. Price, L.C. (1976) Aqueous solubility of petroleum as applied to its origin and primary migration. American Association of Petroleum Geologists Bulletin, 60, 213-244. Roedder, Edwin (1962) Studies of fluid inclusions I: Low temperature application of a dual-purpose freezing and heating stage. Economic Geology, 57, 1045-1061. Roedder, Edwin, Ed. (1968-onward) Fluid Inclusion Research Proceedings of COFFI, vols. 1-5 (1968-1972) published by and available from the editor; vol. 6 (1973-onward) published by University of Michigan Press, Ann Arbor, Michigan. Roedder, Edwin (1970) Application of an improved crushing microscope stage to studies of the gases in fluid inclusions. Schweizerische Mineralogische and Petrographische Mitteilungen,50, 41-58. Roedder, Edwin (1972) The Composition of Fluid Inclusions. United States Geological Survey Professional Paper 440JJ, 164 P. Roedder, Edwin (1979) Fluid inclusion evidence on the environment of sedimentary diagenesis-a review. Society of Exploration Paleontologists and Mineralogists Special Publication 26, Symposium on determination of diagenetic paleotemperatures, 89-107. Roedder, Edwin (1982) Possible Permian diurnal periodicity in NaCl precipitation, Palo Duro basin, Texas. In T.C. Gustavson, et al., eds., Geology and Geohydrology of the Palo Duro Basin, Texas Panhandle, Bureau of Economic Geology. The University of Texas at Austin, Geological Circular 82-7, 101-104. Roedder, Edwin (1984) Fluid inclusions. Reviews in Mineralogy Vol. 12, Mineralogical Society of America, Washington, D.C. (in press). Roedder, Edwin and Bassett, R.L. (1981) Problems in determination of the water content of rock-salt samples and its significance in nuclear waste storage siting. Geology, 9, 525530. Roedder, Edwin and Belkin, H.E. (1977) Preliminary report on study on fluid inclusions in core samples from ERDA No. 9 borehole, nuclear waste site, New Mexico: Report on contract to Sandia Laboratories, June 27, 1977, 29 p. Roedder, Edwin, and Belkin, H.E. (1978) Fluid inclusions in core samples from ERDA No. 9 borehold, WIPP site, New Mexico: Report on contract to Sandia Laboratories, Jan 1, 1978, 31 p. Roedder, Edwin and Belkin, H.E. (1979a) Application of studies of fluid inclusions in Permian Salado salt, New Mexico, to problems of siting the Waste Isolation Pilot Plant. In G.J. McCarthy, Ed. Scientific Basis for Nuclear Waste Management, Vol. l, p. 313-321. Plenum Press, New York. Roedder, Edwin and Belkin, H.E. (1979b) Fluid inclusion study on core samples of salt from the Rayburn and Vacherie Domes, Louisiana. United States Geological Survey Open-file Report 79-1675, 25 p. Roedder, Edwin and Belkin, H.E. (1980a) Migration of fluid inclusions in polycrystalline salt under thermal gradients in the laboratory and in Salt Block II. (abstr.) Proceedings 1980 National Waste Terminal Storage Program Information Meeting, Dec. 9-I1, 1980, Columbus, Ohio. ONWI-212, p. 361-363. Roedder, Edwin and Belkin, H.E. (1980b) Thermal gradient migration of fluid inclusions in single crystals of salt from the Waste Isolation Pilot Plant site (WIPP). In C.J.M. Northrup, Ed. Scientific Basis for Nuclear Waste Management, Vol. 2, p. 453-464. New York, Plenum Press. Roedder, Edwin and Belkin, H.E. (1981) Petrographic study of fluid inclusions in salt core samples from Asse mine, Federal Republic of Germany. United States Geological Survey Openfile Report 81-1128, 32 p. Roedder, Edwin and Bodnar, R.J. (1980) Geologic pressure determinations from fluid inclusion studies. Annual Reviews of Earth and Planetary Science, 8, 263-301. Roedder, Edwin and Chou, I-Ming (1982) A critique of "Brine migration in salt and its implications in the geologic disposal of nuclear waste", Oak Ridge National Laboratory Report 5818, by G.H. Jenks and H.C. Claiborne. United States Geological Survey Open-file Report 82-1131, 31 p. Rosasco, G.J. and Roedder, Edwin (1979) Application of a new Raman microprobe spectrometer to nondestructive analysis of sulfate and other ions in individual phases in fluid inclusions in minerals. Geochimica et Cosmochimica Acta, 43, 1907-1915. Rosasco, G.J., Roedder, Edwin and Simmons, J.H. (1975) Laser-excited Raman spectroscopy for nondestructive partial analysis of individual phases in fluid inclusions in minerals. Science, 190, 557-560. Schneider, Robert and Trask, N.J. (1983) U.S. Geological Survey Research in Radioactive Waste Disposal-Fiscal Year 1981. United States Geological Survey Water Resources Investigations Report 83-4105, 122 p. Schreiber, B.C. and Hsü, K.J. (1980) Evaporites. In G.D. Hobson, Ed. Developments in Petroleum Geology-2, p. 87138. Applied Science Pub., Ltd., London. Shearman, D.J. (1970) Recent halite rock, Baja California, Mexico. Institute of Mining and Metallurgy Transactions, 79, 155-162. Shearman, D.J. (1978) Evaporites of coastal sabkhas; Ancient sabkha deposits; and Halite in sabkha environments; Section 2. In W.E. Dean and B.C. Schreiber, Eds. Marine Evaporites, p. 6-42. Society of Exploration Paleontologists and Mineralogists Short Course Notes No. 4. Shlichta, Paul (1962) Growth, deformation, and defect structure of salt crystals. Geological Society of America Special Paper 88, 597-617. Smith, S.P. and Kennedy, B.M. (1983) The solubility of noble gases in water and in NaCI brine. Geochimica et Cosmochimica Acta, 47, 503-515. Sofer, Z. (1978) The isotopic composition of hydration water in gypsum. Geochimica et Cosmochimica Acta, 42, 1141-1149. Sofer, Z. and Gat, J.R. (1975) The isotopic composition of evaporating brines: Effect of the isotopic activity ratio in saline solutions. Earth and Planetary Science Letters, 26, 179-186. Stewart, D.B., Jones, B.F., Roedder, Edwin and Potter, R.W., II. (1980) Summary of U.S. Geological Survey investigations of fluid-rock-waste reactions in evaporite environments under repository conditions. In Underground Disposal of Radioactive Wastes, Vol. I, p. 335-344. Proceedings IAEA Symposium, Helsinki, 1979: Vienna, Internat. Atomic Energy Agency. Talbot, C.J., Tully, C.P. and Woods, P.J.E. (1982) The structural geology of Boulby (potash) mine, Cleveland, United Kingdom. Tectonophysics, 85, 167-204. Thoma, Klaus and Eckart, Dietrich (1964) Untersuchungen an gashaltigen Mineralsalzen. I. Teil: Bergmännische Untersuchungen. Bergakademie, 16, (11), 674-676 (in German). Urai, J.L. (1983a) Deformation of wet salt rocks. Ph.D. dissertation, Univ. Utrecht, 221 p. Urai, J.L. (1983b) Water assisted dynamic recrystallization and weakening in polycrystalline bischofite. Tectonophysics, 96, 125-157. Valiashko, M.G. (1951) Structural features of deposits of modern halite. L'vov Geologicheskoe Obshchestvo, Mineralogicheskii Sbornik, 5, 65-74. (in Russian). Vil'denberg, Ye. V., Vysotskiy, I.V., Voytov, G.I., and Murogova, R.N. (1978) Gases in salt of the Caspian basin and adjacent districts. Doklady Akademii Nauk SSSR, 239, 14301433 (in Russian; transl. in Doklady Academy of Sciences of the USSR, 239, 179-182, pub. 1980). Wardlaw, N.C. and Schwerdtner, W.M. (1966) Halite-anhydrite seasonal layers in the Middle Devonian Prairie Evaporite Formation, Saskatchewan, Canada. Geological Society of America Bulletin, 77, 331-342. Wilcox, W.R. (1969) Anomalous gas-liquid inclusion movement. Industrial and Engineering Chemistry, 61, 76-77. Yagnik, S.K. (19ß3a) Interfacial stability of migrating brine inclusions in alkali halide single crystals supporting a temperature gradient. Journal of Crystal Growth, 62, 612-626. Yagnik, S.K. (1983b) Thermal-gradient Migration of Brine Inclusions in Salt Crystals. Ph.D. dissertation, Materials and Molecular Research Division, Lawrence Berkeley Laboratory, University of California. NOTES 1 Adapted from Presidential address at the annual meeting of the Mineralogical Society of America, Nov. 1, 1983, Indianapolis, Indiana, and based in part on the results of work performed for the Bureau of Economic Geology of the University of Texas at Austin. 2 Natural secondary inclusions, except occasional planes of very small inclusions, are relatively rare in bedded salt; artificial secondary inclusions, particularly from coring procedures, can be abundant and cause difficulty (Roedder and Belkin, 1981; Roedder and Bassett, 1981). 3 Note that the inclusions in Fig. 11 are between crystals, i.e., intercrystalline inclusions, rather than the intracrystalline inclusions discussed up to this point. 4 Unpublished work of H.E. Belkin and E.L. Libelo, U.S. Geological Survey; manuscript in preparation. 5 The time factor is important and salt fractures very easily under short-term stresses. I have accidentally generated, within seconds, planes of thousands of secondary water inclusions in salt crystals, by thermal fracturing of the surface during too vigorous polishing. 6 LiCl is the only geologically reasonable alternative. 7 Some of the chemical data also cannot be readily explained by this scenario. 8 Wilcox (1969) showed that synthetic liquid inclusions con taining very large bubbles moved down a thermal gradient, but practically all liquid inclusions in natural salt have either a very small bubble or no bubble at all (see also Yagnik, 1983b) 9 The possible mechanisms whereby such fluids might subsequently move (and hence disperse radionuclides from the waste) involve numerous geological parameters and are beyond the scope of this paper. 10 Some have suggested that on migration through salt the fluid inclusion would sweep out trace constituents from the salt, and that this effect might be responsible for some of the inclusion compositional data. Although this process is the basis of the "zone refining" used in commerce, the geological parameters are such that its effects would be negligible. 11 An (admittedly extreme) linear extrapolation from the available experimental data at 1.5°C/cm to the geothermal gradient (assumed to be 15°C/km) would indicate that the inclusions should have moved ~100 meters downward in 300 m.y. This is 6 orders of magnitude larger than the minimum amount of movement that should be readily recognizable on petrographic examination. Manuscript received, February 1, 1984; accepted for publication, February 7, 1984. [var:'startyear'='1984'] [Include:'footer.htm'] |
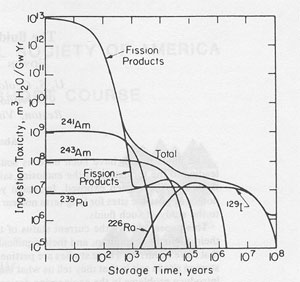

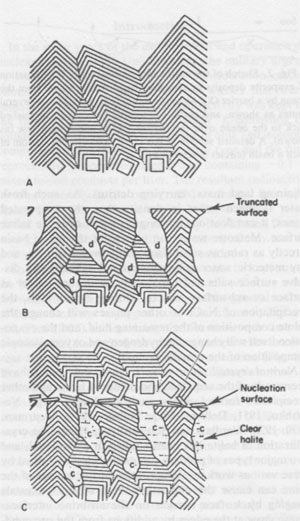
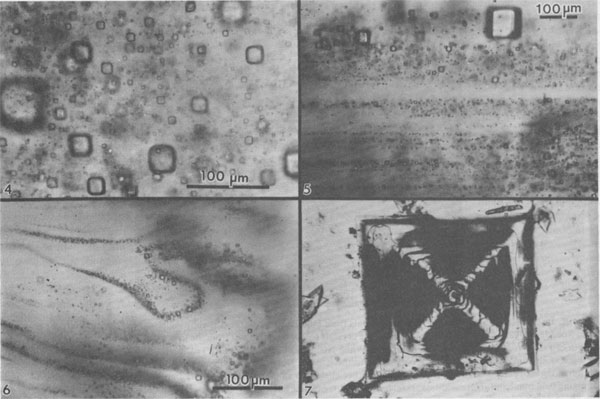
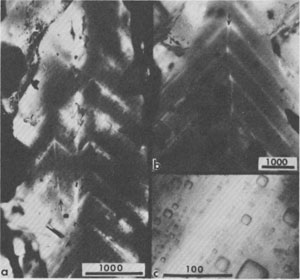
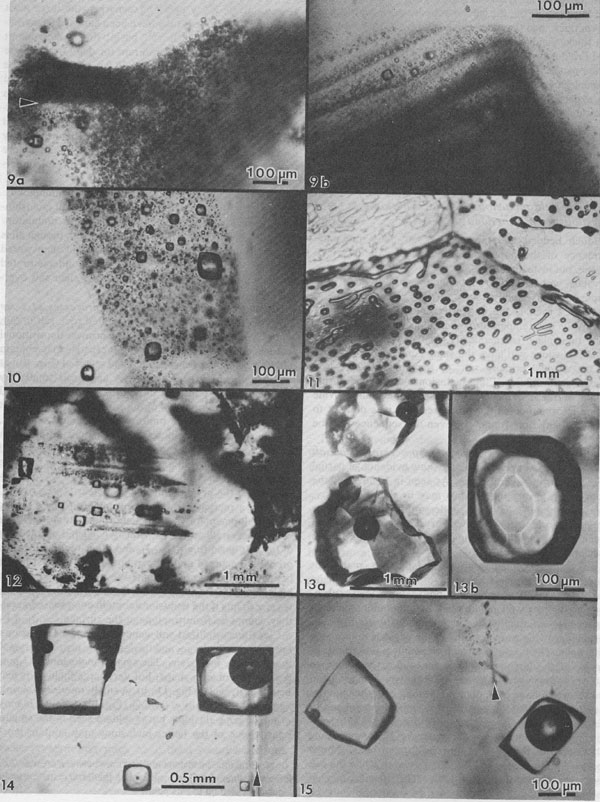
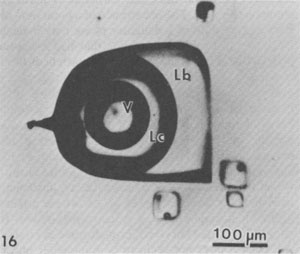
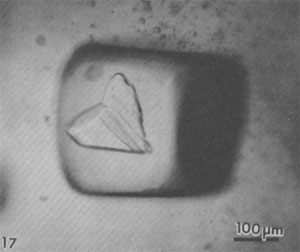
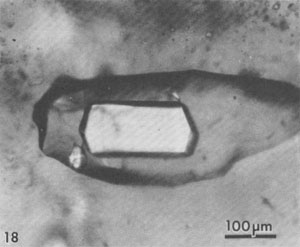
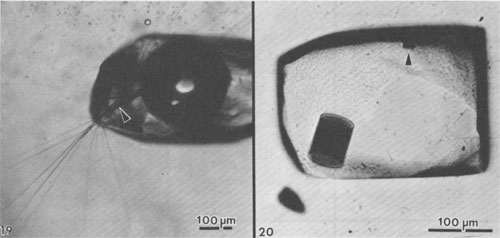
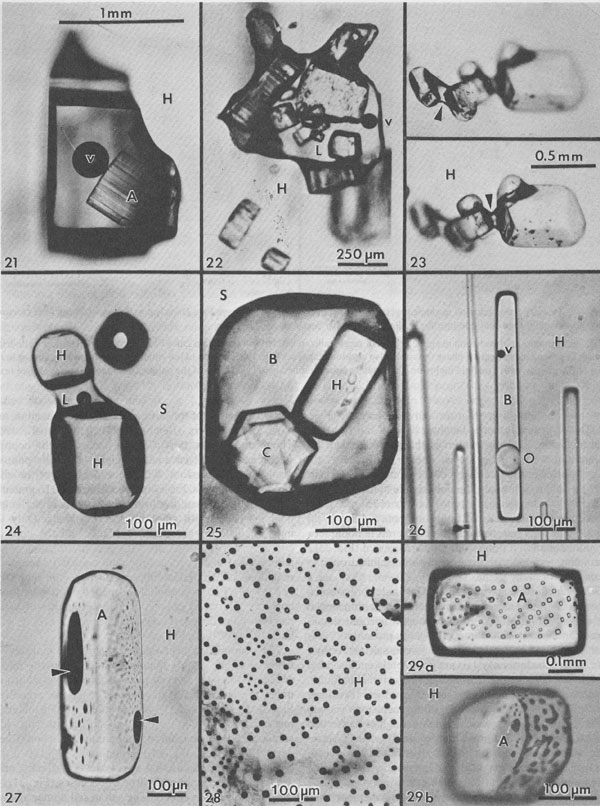
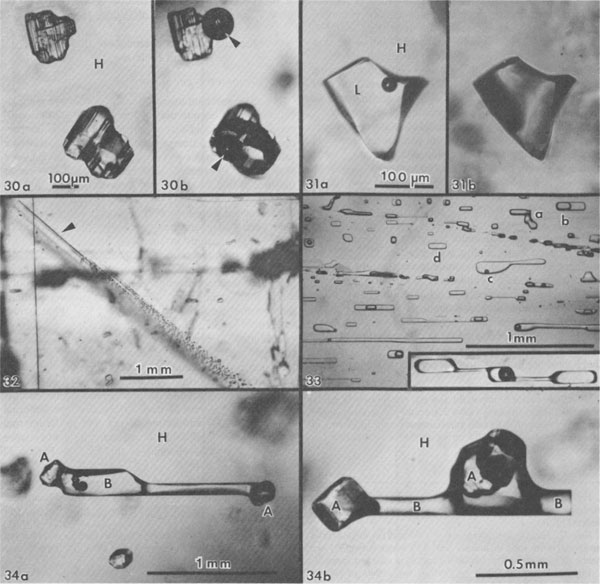
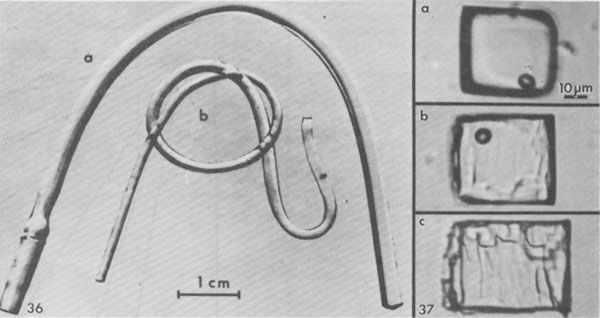 Fig. 36. Synthetic single-crystal rods of halite bent dry (a) and under
water solution (b) at 25°C. From Shlichta (1962).
Fig. 36. Synthetic single-crystal rods of halite bent dry (a) and under
water solution (b) at 25°C. From Shlichta (1962).