|
|
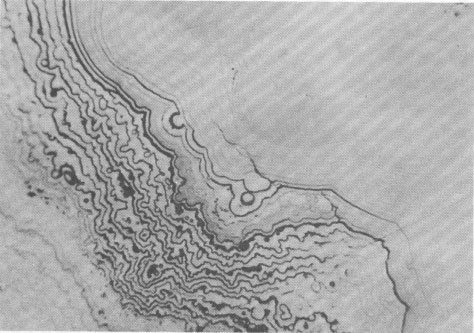
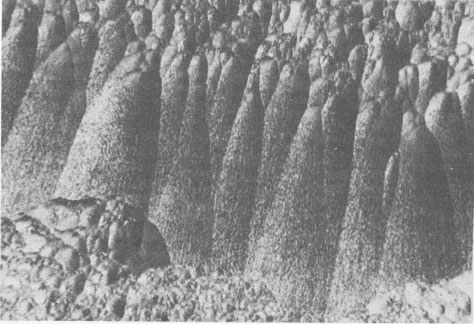
Volume 58, pages 1065-1068, 1973 Hetaerolite from the Rodna Base Metal Ore Deposit: A New Occurrence GEORGE UDUBASA, JOACHIM OTTEMANN, AND GEORGE AGIORGITIS Institute of Mineralogy, University of Heidelberg, and Department of Geosciences, Technical University, Darmstadt, West GermanyAbstract A new occurrence of hetaerolite from Rodna/Rumania is described and detailed mineralogical and chemical investigations undertaken. Introduction The mineral hetaerolite (ZnMn 2O4) which is chemically similar to the mineral hausmannite, was first described from the Sterling Hill deposit, New Jersey, by Moore (1877) and later studied in detail by Palache (1910). Other localities where hetaerolite is known to occur include the Franklin Mine, New Jersey, and Leadville, Colorado (Frondel and Heinrich, 1942). Recently Hewett and Fleischer (1960) have reported hetaerolite from the Contact Mine, Grant County, New Mexico, and the Lucky Cuss Mine, Tombstone, Arizona. They as well as Ramdohr and Frenzel (1956) before them, expressed the opinion that hetaerolite - as a product of weathering of Mn-bearing zinc ores - is much more abundant than previously thought. Now hetaerolite has also been found in the Pb-Zn-deposit at Rodna, Rumania, probably the first locality in Europe. The Rodna deposit belongs to the neogene metallogenetic province of the Carpathian Mountains (Giusca, Cioflica, and Udubasa, 1969) and consists of metasomatic ore-bodies in metasomatic limestones and of injections in andesitic explosion-breccias. The ores consist predominantly of pyrite, sphalerite, pyrrhotite, and galena (Udubasa, 1970). The hetaerolite discussed here was found in cavities within the crystalline limestones where it occurs in a matrix of limonitic ochre together with broken-off fragments of galena crystals, which have been partially coated by cerussite.Macroscopically the hetaerolite is usually stalactitic but occasionally botryoidal in appearance (Fig. 1). Under reflected light it is gray, and similar to magnetite. Its reflectivity measured with a Leitz-microphotometer is 17-19 percent (see Nichol and Phillips, 1965) and decreases considerably under oil immersion. The mineral is weakly pleochroic, even under oil immersion; reflection pleochroism can be observed only along zone or grain-boundaries. Changes in color and reflectivity were nevertheless observed frequently; they were attributed, however, to differences in grain-size and porosity. As a rule homogeneous, coarse grained zones tend to be enclosed by fine-layered ones (Fig. 2), which points to rhythmic separation from a gel. Hetaerolite is clearly anisotropic, but without colorplay. Of interest are the "pyramidal" microstructures observed under crossed nicols (Fig. 3), which are interspersed with fine-grained layers. This feature leads to an increase in anisotropy. Internal reflections were not observed. Experimental Results The hetaerolite was analyzed for Mn, Zn, and Pb with the electron microprobe (Table 1). Spectrally pure metallic manganese, synthesized zincoxide, and stoichiometric galena were used as standards. The corrections were carried out using the X-ray absorption coefficients of Heinrich (1966). Infrared-spectrometric study showed only 1.0 percent OH. Disregarding this small quantity of OH, the chemical formula for hetaerolite from Rodna may be reduced to: (Zn 1.26Pb0.02) Mn1.81O4Hetaerolite coexists locally with a mineral which, based on microprobe analysis (MnO 2, 59.69 percent; PbO, 32.34 percent; ZnO, 5.33 percent), appears related to either coronadite or cesarolite. Its small quantities prevented its study by means of X-ray analysis. Its optical properties could not be determined with exactness. Its reflectivity is greater then of hetaerolite (R = 22-23 percent) and its hardness less. The mineral appears to be weakly anisotropic.
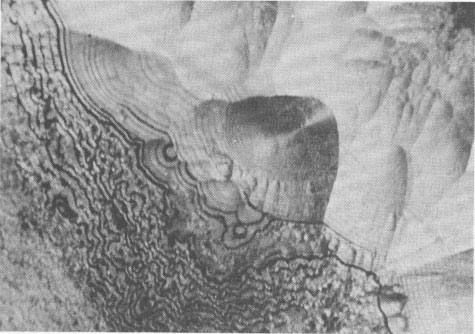
FIG. 2. Fine-layered hetaerolite encrusted with a weakly recrystallized zone of the same mineral. Polished section, oil immersion, magnification 930 X. (a) parallel nicols (b) crossed nicols.
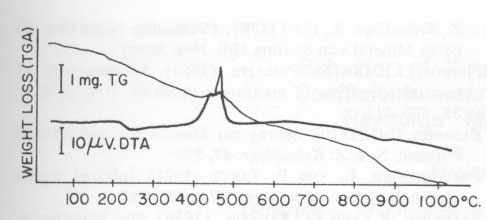
The thermal behavior of hetaerolite was studied with a Mettler DTA-analyzer using a heating-rate of 10°C/min in flowing air. DTA and TGA measurements were obtained simultaneously with this instrument. Hetaerolite shows a double reversible exothermic reaction at 460 and 480°C (Fig. 4). Both peaks possibly point to the oxidation of the manganese in the hetaerolite. The small (irreversible) peak (reaction) at 882°C is similar to the phase-change of hausmannite from the α-form to the ß-form (Agiorgitis, 1969). From this it may be assumed that the structure of hetaerolite already shows a certain amount of order prior to attaining the double peak, i.e., at about 480°C. A subsequent X-ray study of the heating end-product showed only hetaerolite (Table 2). Whether a phase-change from α-hetaerolite to ß-hetaerolite takes place at 882°C, as with hausmannite, is uncertain. However, the thermogravimetric curve permits us to assume a phase-change based on the continuous decrease in weight. The X-ray data for the starting material (I) and the tempered material (II) clearly show (Table 2) a difference in the intensity of the lines as well as a shift in the d-values between the non-tempered and tempered material. The differences in the intensity of the lines are not only the product of a difference in the crystallinity of both substances but possibly also a difference in a 0TABLE 1. Electron Microprobe Analysis of Hetaerolite from Rodna
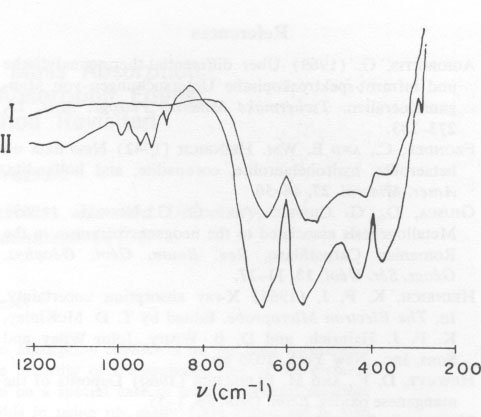
The infrared spectra for substances I and II show similarities in the 700 cm-1 range (Fig. 5). The reduction in the width of the peak at half maximum for heat-tempered substances points to improved crystallinity. The four bands measured correspond to the vibrations v1 (636 cm-1), v2 (540 cm-1), v3 (405 cm-1, 350 cm-1), and v4 (below 200 cm-1). The bands occurring in the frequency range around 900 cm-1 could not be identified with certainty. The fact that other Mn-compounds - for example KMnO 4 - show absorption bands in the 930-900 cm-1 range would tend to indicate that a small fraction of the octahedrally coordinated Mn3+ was oxidized to Mn5+ or Mn7+. This assumption is supported by the observed continuous reduction in weight during thermogravimetric analysis.TABLE 2. Hetaerolite X-ray Data, before Heating (I) and after Heating (II)*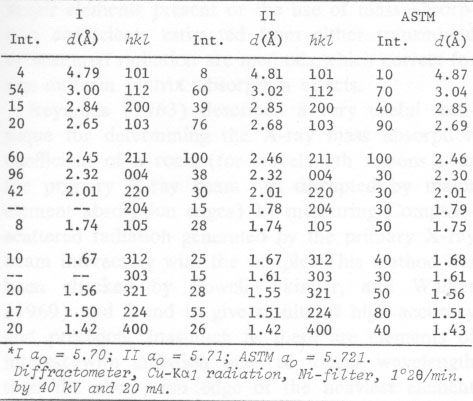
Changes in the crystal structure after heating are also indicated by a split in the v3-vibrations. This split possibly points to a substitution of the octahedrally coordinated Mn3+ by Mn5+, whereas in the case of a substitution of the tetrahedrally coordinated Mn2+ by Mn3+, a separation in the v4-vibrations would be expected. According to Preudhomme and Tarte (1971) only v3 can be split. Therefore, it is possible that the Pb-fraction of hetaerolite from Rodna occupies the octahedral Mn-positions in the crystal structure. The distribution of cations is dependent on temperature and hence can influence changes in the crystallographic axes. Acknowledgments The authors are indebted to Professor P. Ramdohr for encouraging the present investigations. We wish to thank Dr. E. Schot for the English translation. Thanks are extended to the Humboldt-Stiftung for financial support and to the Deutsche Forschungsgemeinschaft for putting the electron microprobe at our disposal. References AGIORGITIS, G. (1969) Über differential-thermoanalytische und infrarot-spektroskopische Untersuchungen von Manganmineralien. Tschermaks Mineral. Petrogr. Mitt. 13, 273-283. FRONDEL, C., AND E. WM. HEINRICH (1942) New data on hetaerolite, hydrohetaerolite, coronadite, and hollandite. Amer. Mineral. 27, 48-56. GIUSCA, D., G. CIOFLICA, AND G. G. UDUBASA (1969) Metallogenesis associated to the neogene volcanism in the Romanian Carpathians. Rev. Roum. Geol. Geophys. Geogr. Ser. Geol. 13, 11-27. HEINRICH, K. F. J. (1966) X-ray absorption uncertainty. In, The Electron Microprobe. Edited by T. D. McKinley, K. F. J. Heinrich, and D. B. Wittry. John Wiley and Sons, Inc., New York. 1035 pp. HEWETT, D. F., AND M. FLEISCHER (1960) Deposits of the manganese oxides. Econ. Geol. 55, 1-55. MOORE, G. E. (1877) Amer. J. Sci. 111, 14, 423. Ref. in Z. Kristallogr. 2, 194 (1878). (Vorläufige Notiz über ein neues Mineral von Sterling Hill, New Jersey). NICHOL, J., AND R. PHILLIPS (1965) Measurements of spectral reflectivity of manganese minerals. Mineral. Mag. 35, 200-213. PALACHE, CH. (1910) Beitrag fur Mineralogie von Franklin Furnace, N. J. Z. Kristallogr. 47, 579. PREUDHOMME, J., AND P. TARTE (1971) Infrared studies of spinels. Spectrochim. Acta, 27A, 845-851. RAMDOHR, P., AND G. FRENZEL (1956) Die Manganerze. XX Congr. Geol. Intern. Symp. sobre yacimientos de manganeso tomo 1, 19-73. UDUBASA, G. G. (1970) Die strukturelle und lithologische Kontrolle der Polymetallagerstätte von Rodna (Ostkarpathen). Rev. Roum. Geol. Geophys. Geogr. Sér. Geol. 14, 13-24.
Manuscript received, March 6, 1973; [var:'startyear'='1973'] [Include:'footer.htm'] |