|
|
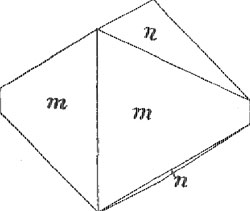
Volume 3, pages 23-27, 1918 SOME MINERALS FROM THE STANLEY ANTIMONY MINE, IDAHO EARL V. SHANNON National Army THE Stanley Mine is located in Gorge Gulch, about one fourth mile from Burke, in the Coeur d'Alene District, Idaho. The vein is unique in that its valuable constituents are antimony and gold, altho it is located in the center of an area characterized by the presence of lead-silver deposits. The vein is lens-like in its character, being exceedingly variable in width and metallic content. The filling of the vein is milky-white quartz, but this gives out in places, the full width of the vein being then pure stibnite. The inclosing rocks are greenish, sericitic slates of the Burke formation, a member of the Algonkian series of sediments known as the Belt Terrane. The mine first began shipments of gold-antimony ore to Granite City, Illinois, in 1906 and maintained a considerable production for some time. The ore was sorted by hand, on the dump, into three products - first-class or shipping ore, consisting of practically pure stibnite; second-class, consisting of scattered small grains of stibnite in quartz; and waste, or the stibnite-free quartz of the gangue. The "second-class" or milling ore was later hauled to the gold mill of the New Jersey Mine near Kellogg and milled. A large part of the gold content was extracted by simple amalgamation and the stibnite was concentrated on Wilfley tables. The mine was later closed but not apparently from lack of ore, as some ten tons of pure stibnite were left for many years in a pile on the dump. Early in 1915, under the stimulus of the then prevailing price of antimony, the mine was reopened and several carloads of hand-sorted stibnite were shipped. The writer while visiting the mine in September of that year was not allowed inside the workings, but had to be content with an inspection of the dumps and ore heaps. The specimens collected at that time form the basis for the following notes. Stibnite. - The sulfide of antimony, stibnite, Sb2S3, forms the principal valuable constituent of the Stanley ores. It is common in large masses of exceptional purity and also scattered in irregular grains and needles thru the white quartz of the gangue. The large masses have a coarse-columnar structure and often show cleavage blades three inches in length. The mineral does not, so far as seen, occur in free crystals of any size and is of no especial mineralogic interest. Sphalerite. - The sulfide of zinc is a constant tho inconspicuous constituent of the vein. It occurs in minute, scattered, crystalline grains of a honey-yellow or light brown color. Gold. - Gold is present in the stibnite to the average value of about $20 per ton. It is rarely visible to the naked eye. One of the ore-sorters showed the writer a specimen of stibnite having small rifts coated with a thin, frost-like film of native gold, the gold evidently being later than the stibnite. Pyrite. - The disulfide of iron, FeS2, is very rare in the vein itself but is abundant in the slaty rocks of the walls, immediately adjacent to the vein. It always occurs in minute brilliant striated and very perfect cubic crystals which seldom exceed one sixteenth inch on an edge. Arsenopyrite. - The sulfarsenide of iron, FeS2.FeAs2, is, like pyrite, a common mineral in the walls of the Stanley vein but does not occur in the filling of the vein proper. It occurs always as perfect and well-defined crystals of a gray to iron-black color and rather dull metallic luster. The crystals, which are evidently replacements of the sericitic rock in which they occur, are slightly larger than those of pyrite in the same specimen and reach an extreme size of one fourth inch. The dark gray color is not a tarnish, as the crystals show the same color on fresh fracture. Penetration twins and sometimes trillings occur. The crystals are easily detached from the matrix but are not well suited for measurement on the reflecting goniometer as all of the faces are deeply striated. The common aspect is like Figs. 1 and 2, the forms recognized being m(110), n(012) and φ(023). Both the pyrite and arsenopyrite appear to have been deposited before the quartz and stibnite of the vein; fragments of the wall rock included in the quartz of the vein contain these minerals.
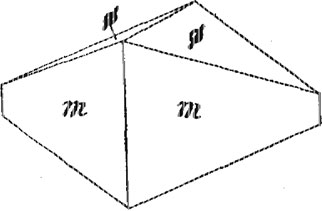
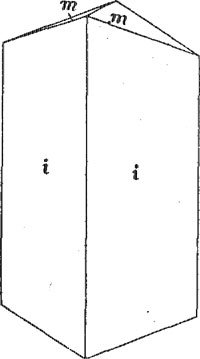
Kermesite. - The red monoclinic oxysulfide of antimony, 2Sb2S3.Sb2O3, occurs as an oxidation product of stibnite in the richest ores, in the form of a blood-red to maroon powder thinly coating cracks in the stibnite. There was not enough of the material for an analysis but the mineral was identified by its color and characteristic blowpipe reactions. No crystals were observed. The mineral is mentioned by Dana1 from Quebec and New Brunswick but not from any locality in the United States. Eakle2 reports cherry-red needles from the Mojave antimony mine, 15 miles north of Mojave, Kern Co., California. Valentinite. - This mineral, which is an orthorhombic trioxide of antimony, Sb2O3, is common in the Stanley ores as coatings on stibnite and on quartz. In color the mineral ranges from pale green to buff, the luster being somewhat waxy. The greenish to brown waxy coatings look very much like cerargyrite or embolite. The buff variety is common both on quartz and on stibnite but the green variety was noted only on the latter. The crusts of valentinite consist of drusy crystals of simple form. These are of small size and have rather dull faces and rounded edges which render them unsuitable for measurement on the reflecting goniometer. One specimen contained rather large clean-cut crystals having the form shown in Fig. 3. The a and c axes are interchanged in the drawing in order to give a clearer idea of the proportions of the crystals. The forms present are m(110) and i(054). The crusts rarely are more than 1/16" in thickness, but surfaces several inches square may be coated with valentinite crystals up to 1/10 inch in length. This mineral, like the preceding one, is apparently rare in the United States.
Cervantite. - Antimony ocher, Sb2O3.Sb2O5. Occurs commonly as thin, canary-yellow coatings on stibnite. The cervantite seems to be a transitory stage and hydrates easily to the white, hydrous oxide described below. Cervantite is easily distinguished from the other minerals of the ore by its color and by the fact that it yields no water in the closed tube. "Stibioferrite." - In small cavities in the quartz from the upper most tunnel of the Stanley Mine, there are thin coatings of a yellow, brownish-yellow or green mineral. This mineral is soluble in hydrochloric acid and the solution reacts for antimony and iron. Some water is given off in the closed tube. The substance thus resembles stibioferrite, a name proposed by Goldsmith for a coating on stibnite from Santa Clara Co., Cal. Volgerite. - During the visit to the Stanley property, it was noted that many of the lumps of stibnite had an outer crust, composed of a soft, earthy material of dirty white color. This material was abundant at the mine, but some preliminary blowpipe trials gave no distinctive reactions and the mineral looked so much like finely triturated quartzite that it was thought to be only a hardened phase of the gouge which accompanies the vein, and only a small fragment was preserved, accidentally included in the box with the other specimens from the locality. Subsequent study of this fragment showed that the material is a hydrous oxide of antimony containing more than twice as much water as is assigned to stibiconite. The mineral characteristically occurs as shells around the stibnite, the coatings varying from one eighth inch to two inches in thickness. Coatings thinner than one eighth inch usually consist of cervantite, and the whitish mineral seems to result from the further oxidation and hydration of this. The mineral agrees in some particulars with volgerite and is tentatively referred to this species. The material of the interior of the crusts is compact, somewhat translucent, has a faint resinous luster, and a pale grayish-brown color. Toward the exterior of the crust this grades into lusterless, friable earthy material of a dirty white color. So far as could be learned, these two varieties are composed of the same substance, the friable material resulting from the mechanical disintegration of the compact variety. Only a small portion was available for analysis and after the iron and water had been determined, the precipitated antimony sulfide was accidentally lost, without its weight being found. Since an analysis of an antimony oxide is scarcely complete with determinations of neither antimony nor oxygen, the investigation was put aside until more material could be obtained. The additional material has not been forthcoming, however, and since the writer will probably be unable to revisit the locality, these notes are recorded for the benefit of those who may have obtained specimens of the same mineral from this or other localities. The hardness of the mineral is probably about 3.5 as it scratches calcite easily but does not scratch fluorite. The specific gravity, determined on approximately 0.6 g. of selected small fragments, is 3.082 at 20° C. The mineral contains 12.6% of water, and 1.4% Fe2O3. It is completely soluble in hot concentrated hydrochloric acid. After the iron and antimony were removed from the solution, it was carefully tested and no other elements were found. The mineral is evidently a hydrous oxide of antimony, pure except for the presence of a small amount of iron. The powder is dirty brownish to greenish-white. All of the water is given off below a red heat, the powder when dehydrated becoming snowy-white. Slightly above a red heat some oxygen is given off, the higher oxide, Sb2O5, apparently passing into Sb2O4. The name volgerite was given by Dana to the compound Sb2O5.5H2O, described by Volger, the so-called cumengite, Sb2O5.4H2O, being included. The water content of the mineral described above is too low to conform with either formula. Such colloid minerals vary widely in water content, however, and until the composition is more definitely established, the mineral may best be referred to as volgerite. NOTES 1 Dana, E. S., System of Mineralogy, p. 107. 2 Eakle, A. S., Minerals of Calif., Bull. Cal. State Min, Bureau, No. 67, p. 43. [var:'startyear'='1918'] [Include:'footer.htm'] |