|
|
Volume 65, pages 1-7, 1980 Summary of recommendations of AIPEA nomenclature committee on clay minerals S. W. BAILEY, CHAIRMAN 1Department of Geology and Geophysics Introduction Because of their small particle sizes and variable degrees of crystal perfection, it is not surprising that clay minerals proved extremely difficult to characterize adequately prior to the development of modern analytical techniques. Problems in characterization led quite naturally to problems in nomenclature, undoubtedly more so than for the macroscopic, more crystalline minerals. The popular adoption in the early 1950s of the X-ray powder diffractometer for clay studies helped to solve some of the problems of identification. Improvements in electron microscopy, electron diffraction and oblique texture electron diffraction, infrared and DTA equipment, the development of nuclear and isotope technology, of high-speed electronic computers, of Mössbauer spectrometers, and most recently of the electron microprobe and scanning electron microscope all have aided in the accumulation of factual information on clays. This, in turn, should facilitate eventual agreement on the nomenclature of clays. Probably the earliest attempt by clay scientists to reach agreement on nomenclature and classification on an international basis was at the International Soil Congress held in Amsterdam in 1950 (Brindley et al., 1951). Since that time national Nomenclature Committees have been established in many countries. Recommendations from these national groups have been considered every three years at the International Clay Conferences, first by the Nomenclature Sub-Committee of CIPEA (Comité International Pour l'Étude des Argiles) and since 1966 by the Nomenclature Committee of AIPEA (Association Internationale Pour l'Étude des Argiles). These international committees in turn have worked closely with the Commission on New Minerals and Mineral Names of the IMA (International Mineralogical Association). This summary of the recommendations made to date by the international nomenclature committees has been prepared in order to achieve wider dissemination of the decisions reached and to aid clay scientists in the correct usage of clay nomenclature. Some of the material in the present summary has been taken from an earlier summary by Bailey et al. (1971a). Classification Agreement was reached early in the international discussions that a sound nomenclature is necessarily based on a satisfactory classification scheme. For this reason, the earliest and most extensive efforts of the several national nomenclature committees have been expended on classification schemes. Existing schemes were collated and discussed (see Brown, 1955, Mackenzie, 1959, and Pedro, 1967, for examples), symposia were held at national meetings, and polls were taken of clay scientists in 32 countries as to their preferences. Armed with these data, the international representatives have been able to agree upon most features of a broadly based scheme for the phyllosilicates as a whole (Mackenzie, 1965a,b; Brindley, 1967). Table 1 gives the classification scheme in its present form. The phyllosilicates are divided into groups, each containing dioctahedral and trioctahedral subgroups. Each sub-group in turn is divided into mineral species. This subdivision corresponds to successive stages of refinement in the identification process. It is anticipated that the precise definitions of the groups and sub-groups and their names will evolve and change with time. This table differs from previously published versions in two respects. Smectite has now been accepted as the group name for clay minerals with layer charge between 0.2 and 0.6 per formula unit. This decision, made at the 1975 Mexico City meeting (Brindley and Pedro, 1976), was based on increased usage world-wide of this name rather than of the alternate dual name of montmorillonite-saponite for the group. Dual names still exist for the kaolinite-serpentine and pyrophyllite-talc groups.
Table 1. Classification scheme for phyllosilicates related to clay minerals
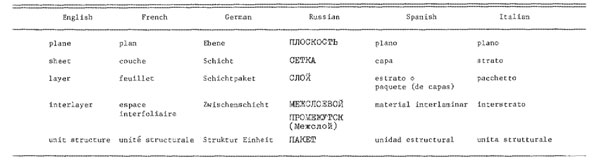
* Only a few examples are given Suggested names of kandite and septechlorite for the kaolin and serpentine minerals, respectively, have not been approved by the AIPEA Committee, and should not be used. The second change is to treat chlorite as consisting of a 2: 1 layer plus an interlayer hydroxide sheet, rather than as a 2: 1: 1 or 2:2 layer type. This emphasizes the similarity of chlorite to other clay minerals containing interlayer material (Brindley and Pedro, 1972). Definition of phyllosilicate Table 1 assumes a specific definition of a phyllosilicate (or layer silicate). This definition was discussed most recently at the AIPEA Nomenclature Committee meeting held in Madrid in 1972, at which a 1969 definition was modified. The present definition (Brindley and Pedro, 1972) states "Clay minerals belong to the family of phyllosilicates and contain continuous two-dimensional tetrahedral sheets of composition T2O5 (T = Si, Al, Be, ...) with tetrahedra linked by sharing three comers of each, and with the fourth corner pointing in any direction. The tetrahedral sheets are linked in the unit structure to octahedral sheets, or to groups of coordinated cations, or individual cations." The present definition is based on the nature of the silicate parts of the structure, and does not include previous requirements of weaker interlayer bonding or of certain resultant physical properties. Thus, it does not require a category of "pseudo-layer silicates" for minerals, such as palygorskite and sepiolite, that do not possess marked basal cleavages. The criterion of a continuous tetrahedral sheet does exclude "quasi-layer silicates," such as astrophyllite, lamprophyllite, bafertisite, and haradaite, in which 5-fold or 6-fold coordinated groups interrupt the continuity of the tetrahedral net. Standardization of structural terms At the 1975 Mexico City meeting the AIPEA Nomenclature Committee noted that "lattice" and "structure" continue to be misused by authors and speakers. A "lattice" is not synonymous with "structure," but is a uniform distribution of points in space (e.g. the 14 Bravais lattices). The terms "layer lattice" and "Schichtgitter" are incorrect and should not be used. Layer structure, layer silicate, and phyllosilicate are acceptable terms (Brindley, 1967; Brindley and Pedro, 1976). In 1972 the Committee agreed upon usage of the terms "plane," "sheet," "layer," "unit structure," and their equivalents in other languages (Brindley and Pedro, 1972). Recommended usage is as a single plane of atoms, a tetrahedral or octahedral sheet, and a 1: 1 or 2: 1 layer. Thus, plane, sheet, and layer refer to increasingly thicker arrangements. A sheet is a combination of planes and a layer is a combination of sheets. In addition, layers may be separated from one another by various interlayer materials, including cations, hydrated cations, organic molecules, and hydroxide octahedral groups and sheets. The total assembly of a layer plus interlayer material is referred to as a unit structure. Table 2 lists the equivalent terms in other languages, as modified at the 1978 Oxford meeting.
Table 2. Structural terms of reference and their equivalents indifferent
languages
The terms "talc layer" and "brucite sheet" are not suitable for describing the component parts of the chlorite structure, because the minerals talc and brucite permit very little substitution of Mg by Al, which is an essential feature of trioctahedral chlorites. It is recommended that 2:1 layer be used in place of "talc layer" and hydroxide sheet or interlayer sheet in place of "brucite sheet" (Brindley and Pedro, 1972). It is permissible to write brucite-like or brucitic or gibbsite-like or gibbsitic if one wishes to specify the trioctahedral or dioctahedral nature of the interlayer. Attention is drawn also to the report of the IMAIUCr Joint Committee on Nomenclature (Bailey, 1977), in which the following recommendations will be of special interest to clay scientists. These recommendations have been approved by the AIPEA Nomenclature Committee. (1) Polytypism is defined as "the phenomenon of the existence of an element or compound in two or more layer-like crystal structures that differ in layer stacking sequences. The layers need not be crystallographically identical, but should be similar. Polytypism differs from polymorphism (in the present and strict definition of the latter term) in permitting small differences in chemical composition between structures, not to exceed 0.25 atoms per formula unit of any constituent element. Layer structures that differ from one another by more than this amount are to be called polytypoids rather than polytypes." (2) "In general, polytypes should not receive individual mineral names. Instead, a set of related polytypes should be designated by a single name followed by a structural symbol suffix that defines the layer stacking differences." A recommended system of structural symbols is described in the report. (3) "Polytype mineral names already in existence that have international acceptance and serve a useful function need not be discarded. Decision on retention of individual names should be the responsibility of the IMA Commission on New Minerals and Mineral Names." (4) "It is recommended that X, Y, Z, or [1001, [010], [001] be used for directions of crystallographic axes and a, b, c for the repeat distances along these axes." Interstratifications and non-crystalline materials No general agreement has been reached yet as to preferred terminology for interstratified minerals, except that the material should be characterized fully as to degree of regularity or irregularity of the interstratification and that it should be described in terms of the nature and ratios of the component layers. The best descriptive terms for those layers are still in question. At the 1972 Madrid meeting the Committee recommended that specific names not be given to poorly defined materials, such as irregularly interstratified systems, to imperfect structures (e.g. deweylite and aquacreptite), or to non-crystalline constituents. Special names can be given to regularly interstratified minerals, subject to acceptance by the AIPEA Nomenclature Committee and the IMA Commission on New Minerals and Mineral Names (Brindley and Pedro, 1972). Names already in the literature at that time were rectorite for a regular 1: 1 interstratification of dioctahedral paragonite-smectite (Brown and Weir, 1963), corrensite for a regular 1: 1 interstratification of trioctahedral chlorite-"swelling chlorite" (Lippmann, 1954), tosudite for a regular l: 1 interstratification of dioctahedral chlorite-smectite (Frank-Kamenetskii et al., 1963; Shimoda, 1969), and aliettite for a regular l: 1 interstratification of trioctahedral talc-saponite (Veniale and van der Marel, 1969). The IMA Commission on New Minerals and Mineral Names has disapproved the name sangarite for a regular l: 1 interstratification of trioctahedral chlorite-vermiculite (Drits and Kossovskaya, 1963), and has approved the name tarasovite for a regular 3: 1 interstratification of dioctahedral mica-smectite (Lazarenko and Korolev, 1970). The AIPEA Nomenclature Committee has taken no action as yet on specific names for regular interstratifications. The CIPEA Nomenclature Sub-Committee at its Jerusalem meeting (Brindley, 1967) agreed unanimously that the term "non-crystalline" is preferable to the commonly used term "amorphous." It was recommended strongly that specific names not be given to newly discovered non-crystalline minerals, but that they be described so far as possible in terms of their chemical composition. Names may be chosen later if it becomes apparent that particular ranges of chemical composition exist for these minerals. Specific phyllosilicate names Dioctahedral chlorite The Committee has recommended (Brindley and Pedro, 1970) that the chlorite group be subdivided into the three sub-groups dioctahedral chlorite, di,trioctahedral chlorite, and trioctahedral chlorite (Table l). Dioctahedral chlorite is dioctahedral in both the 2: 1 layer and the interlayer hydroxide sheet. An example is donbassite (Lazarenko, 1940). Trioctahedral chlorite is trioctahedral in both octahedral sheets. A di,trioctahedral chlorite is dioctahedral in the 2: 1 layer but trioctahedral in the interlayer sheet. Cookeite and sudoite are examples, with cookeite being Li-rich and sudoite Li-poor. No examples are known as yet of chlorites with trioctahedral 2: 1 layers but dioctahedral interlayers. Trioctahedral chlorite At the 1978 Oxford meeting the AIPEA Nomenclature Committee adopted the suggestion of Bayliss (1975) for simplification of chlorite nomenclature. Trioctahedral chlorites should be named according to the dominant divalent octahedral cation present. Recommended species names are clinochlore for Mg-dominant [end member = (Mg5Al)(Si3Al)O10(OH)8], chamosite for Fe2+-dominant [end member = (Fe52+Al)(Si3Al)O10(OH)8], nimite for Ni-dominant [end member = (Ni5Al)(Si3Al)O10(OH)8], and pennantite for Mn2+-dominant [end member = (Mn52+Al)(Si3Al)O10(OH)8]. All other species and varietal names should be discarded because arbitrary subdivisions according to octahedral and tetrahedral compositions have been shown to have little or no structural significance. Tetrahedral compositions and trivalent octahedral cations are not considered in the recommended species names, nor is the distribution of octahedral cations between the 2: 1 layer and the interlayer. Adjectival modifiers, such as those of Schaller (1930), may be used to indicate either important octahedral cations other than the dominant cation or unusual tetrahedral compositions. Bayliss (1975) gives modifiers appropriate for many of the chlorite species listed in other nomenclature systems. Imogolite The Committee at its 1969 Tokyo meeting (Brindley and Pedro, 1970) approved the name imogolite for a hydrous aluminosilicate having a fine thread-like morphology and the diffraction characteristics described by Wada and Yoshinaga (1969) and by others. Halloysite The 1975 AIPEA Nomenclature Committee reviewed the several terminologies in use for the less hydrous and the more hydrous forms of halloysite. The terms halloysite(7A) and halloysite(10A) were recommended for general usage as being least ambiguous (Brindley and Pedro, 1976). The term endellite should not be used. Celadonite The 1978 AIPEA Nomenclature Committee has defined celadonite as a dioctahedral mica of ideal composition KMgFe3+Si4O10(OH)2 but allowing a tetrahedral Al (or Fe3+) range of 0.0 to about 0.2 atoms per formula unit. Substantial octahedral variations from this formula can be described by adjectival modifiers, such as aluminian celadonite or ferroan celadonite. Further characteristics of celadonite are d(060) < 1.510A and sharp infrared spectra, as described by Buckley et al. (1978). There is an area of potential overlap of celadonite and glauconite analyses between about AlIV = 0.17 to 0.20 atoms. For compositions near this boundary and for cases where analytical errors or impurities are suspected, application of the other identification criteria are especially important. Glauconite Buckley et al. (1978) have shown that with careful purification and modem analytical techniques there is little or no overlap between celadonite and glauconite compositions and that the two minerals can be differentiated also by d(060) values and infrared spectra. The 1978 AIPEA Nomenclature Committee has defined glauconite as an Fe-rich dioctahedral mica with tetrahedral Al (or Fe3+) usually greater than 0.2 atoms per formula unit and octahedral R3+ correspondingly greater than 1.2 atoms. A generalized formula is K(R1.333+R0.672+)(Si3.67Al0.33)O10(OH)2 with Fe3+ > Al and Mg > Fe2+ (unless altered). Further characteristics of glauconite are d(060) > 1.510A and (usually) broader infrared spectra than celadonite, as described by Buckley et al. (1978). The species glauconite is single-phase and ideally is non-interstratified. Mixtures containing an iron-rich mica as a major component can be called glauconitic. Specimens with expandable layers can be described as randomly interstratified glauconite-smectite. Mode of origin is not a criterion, and a green fecal pellet in a marine sediment that meets the definition for celadonite should be called celadonite. Miscellaneous Attention is drawn here to recommendations made by other nomenclature committees, although not specifically considered by the AIPEA Nomenclature Committee. The name berthierine has priority for the Fe-rich 1: 1 type layer silicate having appreciable tetrahedral Al and commonly found in ironstones and iron formations. Brindleyite is the Ni-analogue of berthierine. The name chamosite has priority for a 2: 1 chlorite of composition similar to berthierine (Orcel et al., 1949). The name clintonite has priority over other species names (xanthophyllite, seybertite, brandisite, valuevite) for the Li-poor, Ba-poor trioctahedral brittle mica. All of these are so similar in crystallography, chemical composition, and mode of origin that only a single species name is justified (Forman et al., 1967). Bityite (Li,Be-rich), anandite (Ba,Fe-rich), and kinoshitalite (Ba,Mg-rich) appear to be other valid trioctahedral brittle micas species (Schaller et al., 1967; Pattiaratchi et al., 1967; Yoshii et al., 1973). Ephesite, described originally as a Li-Na brittle mica (Schaller et al., 1967), is described better as a true mica with a layer charge per formula unit of unity. The name palygorskite has priority over attapulgite for the mineral with ribbon-like structure in which the ribbons have a width of two pyroxene-like chains (Bailey et al, 1971b). The name anauxite has been discredited. It is a mixture of components, of which the kaolin component is true kaolinite (Langston and Pask, 1968; Allen et al., 1969; Bailey and Langston, 1969). Medmontite is a mixture of chrysocolla and mica, and the name should be discarded (Chukhrov et al., 1968, 1969; Fleischer, 1969a). Nimite is the preferred term for the trioctahedral chlorite with Ni dominant (Hiemstra and de Waal, 1968a). Specimens previously termed schuchardite have Ni < Mg (Fleischer, 1969b), and should be called nickeloan clinochlore. Brindley and De Souza (1975) also have shown that some "schuchardites" are transitional between chlorite and vermiculite. Caryopilite is the preferred term for a 1:1 layer type mineral that is the Mn2+-analogue of greenalite. The name bementite, sometimes used for the former mineral, has priority for a Mn-rich mineral that belongs to the friedelite group of minerals and is not a layer silicate (Kato, 1963). The name rectorite has priority over allevardite for a regular 1: 1 interstratification of paragonite-smectite (Brown and Weir, 1963). Sungulite and kolskite are mixtures of lizardite and sepiolite, and the names should be discarded (Ivanova et al., 1973). Alushtite is a mixture of dickite and hydrous mica, and the name should be discarded (Logvinenko and Frank-Kamenetskii, 1955). Some specimens that have been called alushtite have been identified later as tosudite (Frank-Kamenetskii et al., 1963). Deweylite is a mixture in variable proportions of a disordered form of talc (kerolite) and a disordered form of serpentine. Both components have excess water, probably associated with unbalanced surface bonds. The name is useful only as a field term (Bish and Brindley, 1978). Kerolite is a varietal name for a mineral close to talc in composition and structure but with highly random layer stacking and an enlarged basal spacing of about 9.6A due to misfitting layers. R32+(Si2O5)2(OH)2·nH2O with n ~ 0.8-1.2 (Brindley et al., 1977). Pimelite is a Ni-analogue of kerolite with Ni > Mg (Maksimovic, 1966; Brindley et al., 1979). Nepouite is a Ni-analogue of lizardite (Glasser,1907; Maksimovic, 1973; Brindley and Wan, 1975). New names for layer silicate minerals approved recently by the IMA Commission on New Minerals and Mineral Names are listed below: hendricksite, a trioctahedral Zn-rich mica (Frondel and Ito, 1966; Frondel and Einaudi, 1968) willemseite, a Ni-analogue of talc (Hiemstra and de Waal, 1968b) pecoraite, a Ni-analogue of clinochrysotile (Faust et al., 1969) Mn-sepiolite, Mn-palygorskite, Mn-ferrisepiolite, Mn-ferropalygorskite (Semenov, 1969) chernykhite, a dioctahedral V,Ba,Na-rich mica (Ankinovich et al., 1972) kellyite, a Mn2+ - analogue of amesite (Peacor et al., 1974) swinefordite, a Li,Al,Mg-rich smectite intermediate between dioctahedral and trioctahedral (Tien et al., 1975) baumite, a Mn,Fe,Zn-rich serpentine (Frondel and Ito, 1975) masutomilite, a Mn2+-analogue of zinnwaldite (Harada et al., 1976) yofortierite, a Mn2+-analogue of palygorskite (Perrault et al., 1975) falcondoite, a Ni-analogue of sepiolite with Ni > Mg (Springer, 1976) ferripyrophyllite, a Fe3+-analogue of pyrophyllite (Chukhrov et al., 1979). Several layer silicates incorporating interlayer metallic elements have been recognized recently. Chapmanite and bismutoferrite have 1: 1 layers with Si in the tetrahedral sheet and Fe3+ in the octahedral sheet. The surface hydroxyl groups of the octahedral sheet are replaced by oxygens, and Sb and Bi (in chapmanite and bismutoferrite, respectively) are in the interlayer space (Zhoukhlistov et al., 1974; Zhoukhlistov and Zvyagin, 1977). Surite is a smectite having a defect, cerussite-like lead carbonate interlayer (Hayase et al., 1978).
References Allen, V. T., Fahey, J. J., and Ross, M. (1969) Kaolinite and anauxite in the lone Formation. Am. Mineral., 54, 206-211. Ankinovich, S. G., Ankinovich, E. A., Rozhdestvenskaya, I. V., and Frank-Kamenetskii, V. A. (1972) Chernykhite, a new barium-vanadium mica from northwestern Karatau. (in Russian) Zap. Vses. Mineral. Obshch., 101, 451-458. Bailey, S. W. (1977) Report of the I.M.A.-I.U.Cr. Joint Committee on Nomenclature. Am. Mineral., 62, 411-415. Bailey, S. W., Brindley, G. W., Johns, W. D., Martin, R. T., and Ross, M. (1971 a) Summary of national and international recommendations on clay mineral nomenclature. Clays & Clay Minerals, 19, 129-132. Bailey, S. W., Brindley, G. W., Johns, W. D., Martin, R. T., and Ross, M. (1971b) Clay Mineral Society. Report of nomenclature committee 1969-1970. Clays & Clay Minerals, 19, 132-133. Bailey, S. W. and Langston, R. B. (1969) Anauxite and kaolinite structures identical. Clays & Clay Minerals, 17, 241-243. Bayliss, P. (1975) Nomenclature of the trioctahedral chlorites. Can. Mineral., 13, 178-180. Bish, D. L. and Brindley, G. W. (1978) Deweylites, mixtures of poorly crystalline hydrous serpentine and talc-like minerals. Mineral. Mag., 42, 75-79. Brindley, G. W. (1967) CIPEA Nomenclature Sub-Committee, Minutes of meetings held June 20 and 23 (1966), Jerusalem, Israel. Proc. Internat. Clay Conf. 1966 II, xxvii-xxix. Brindley, G. W., Bish, D. L., and Wan, H.-M. (1977) The nature of kerolite, its relation to talc and stevensite. Mineral. Mag., 41, 443-452. Brindley, G. W., Bish, D. L., and Wan, H.-M. (1979) Compositions, structures, and properties of nickel-containing minerals in the kerolite-pimelite series. Am. Mineral., 64, 615-625. Brindley, G. W. and De Souza, J. V. (1975) Nickel-containing montmorillonites and chlorites from Brazil, with remarks on schuchardite. Mineral. Mag., 40, 141-152. Brindley, G. W., MacEwan, D. M. C., Caillere, S., Correns, C. W., Favajee, J. Ch. L., and Grim, R. E. (1951) The nomenclature of clay minerals. Clay Minerals Bull., 1, 194-195. Brindley, G. W. and Pedro, G. (1970) Report of the AIPEA Nomenclature Committee. AIPEA Newsletter No. 4, 3-4. Brindley, G. W. and Pedro, G. (1972) Report of the AIPEA Nomenclature Committee. AIPEA Newsletter No. 7, 8-13. Brindley, G. W. and Pedro, G. (1976) Meeting of the Nomenclature Committee of A.I.P.E.A., Mexico City, July 21, 1975. AIPEA Newsletter No. 12, 5-6. Brindley, G. W. and Wan, H.-M. (1975) Compositions, structures, and thermal behavior of nickel-containing minerals in the lizardite-nepouite series. Am. Mineral., 60, 861-871. Brown, G. (1955) Report of the Clay Minerals Group Sub-Committee on nomenclature of clay minerals. Clay Minerals Bull., 2, 294-302. Brown, G. and Weir, A. H. (1963) The identity of rectorite and allevardite. Proc. Internat. Clay Conf 1963, Stockholm, 1, 27-35. Buckley, H. A., Bevan, J. C., Brown, K. M., Johnson, L. R., and Farmer, V. C. (1978) Glauconite and celadonite: two separate mineral species. Mineral. Mag., 42, 373-382. Chukhrov, F. V., Zvyagin, B. B., Drits, V. A., Gorshkov, A. I., Ermilova, L. P., Goilo, E. A., and Rudnitskaya, E. S. (1979) The ferric analogue of pyrophyllite and related phases. Proc. Internal. Clay Conf. 1978, Oxford, 55-64. Chukhrov, F. V., Zvyagin, B. B., Ermilova, L. P., Gorshkov, A. I., and Rudnitskaya, E. S. (1969) The relation between chrysocolla, medmontite, and copper-halloysite. Proc. Internat. Clay Conf. 1969, Tokyo, 1, 141-150. Chukhrov, F. V., Zvyagin, B. B., Gorshkov, A. I., Ermilova, L. P., and Rudnitskaya, E. S. (1968) The nature of medmontite. (in Russian) Izv. Akad Nauk SSSR, Ser. Geol. 67-71. Drits, V. A. and Kossovskaya, A. G. (1963) Sangarite, a new clay mineral with ordered mixed-layer structure. (in Russian) Dokl. Akad. Nauk SSSR, 151, 934-937. Faust, G. T., Fahey, J. J., Mason, B. and Dwornik, E. J. (1969) Pecoraite, Ni6Si4O10(OH)8, nickel analog of clinochrysotile, formed in the Wolf Creek meteorite. Science, 165, 59-60. Fleischer, M. (1969a) New mineral names. Am. Mineral., 54, 990994. Fleischer, M. (1969b) New mineral names. Am. Mineral., 54, 1739-1742. Forman, S. A., Kodama, H., Maxwell, J. A. (1967) The tri octahedral brittle micas. Am. Mineral., 52, 1122-1128. Frank-Kamenetskii, V. A., Logvinenko, N. V., and Drits, V. A. (1963) A dioctahedral mixed-layer clay mineral, tosudite. (in Russian) Zap. Vses. Mineral. Obshch., 92, 560-565. Frondel, C. and Einaudi, M. (1968) Zinc-rich micas from Sterling Hill, New Jersey. Am. Mineral., 53, 1752-1754. Frondel, C. and Ito, J. (1966) Hendricksite, a new species of mica. Am. Mineral, 51, 1107-1123. Frondel, C. and Ito, J. (1975) Zinc-rich chlorites from Franklin, New Jersey. Neues Jahrb. Mineral. Abh., 123, 111-115. Glasser, E. (1907) Sur une espèce minérale nouvelle, la népouite, silicate hydraté de nickel et de magnésie. Bull. Soc. fr. Mineral., 30, 17-28. Harada, K., Honda, M., Nagashima, K., and Kanisawa, S. (1976) Masutomilite, manganese analogue of zinnwaldite, with special reference to masutomilite-lepidolite-zinnwaldite series. Mineral. J., 8, 95-109. Hayase, K., Dristas, J. A., Tsutsumi, S., Otsuka, R., Tanabe, S., Sudo, T., and Nishiyama, T. (1978) Surite, a new Pb-rich layer silicate mineral. Am. Mineral., 63, 1175-1181. Hiemstra, S. A. and de Waal, S. A. (1968a) Nickel minerals from Barberton. II. Nimite, a nickelian chlorite. Nat. Inst. Met. (South Africa) Res. Rep., 344, 1-10.* Hiemstra, S. A. and de Waal, S. A. (1968b) Nickel minerals from Barberton. III. Willemseite, a nickelian talc. Nat. Inst. Met. (South Africa) Res. Rep. 353, 1-14.* Ivanova, V. P., Kasatov, B. K., and Moskaleva, V. N. (1973) Thermographic studies of serpentines on heating them to 1400°. (in Russian) Zap. Vses. Mineral. Obshch., 102, 3-15. Kato, T. (1963) New data on the so-called bementite. J. Mineral. Soc. Japan, 6, 93-103 (in Japanese). Langston, R. B. and Pask, J. A. (1968) The nature of anauxite. Clays & Clay Minerals, 16, 425-436. Lazarenko, E. K. (1940) Donbassites, a new group of minerals from the Donetz Basin. (in Russian) C. R. Acad. Sci. URSS, 28, 519-521. Lazarenko, E. K. and Korolev, Yu. M. (1970) Tarasovite, a new dioctahedral ordered interlayer mineral. (in Russian) Zap. Vses. Mineral. Obshch., 99, 214-224. Lippmann, F. (1954) Über einen Keuperton von Zaisersweiher bei Maulbronn. Heidelb. Beitr. Mineral. Petrog., 4, 130-134. Logvinenko, L. V. and Frank-Kamenetskii, V. A. (1955) On the so-called alushtite. (in Russian) Dokl. Akad. Nauk SSSR, 105, 554-557. Mackenzie, R. C. (1959) The classification and nomenclature of clay minerals. Clay Minerals Bull., 4, 52-66. Mackenzie, R. C. (1965a) Report of chairman on meeting of Nomenclature Sub-Committee of CIPEA. Proc. Internat. Clay Conf. 1963, Stockholm, Sweden, 2, 439-442. Mackenzie, R. C. (1965b) Nomenclature Sub-Committee of C.I.P.E.A. Clay Minerals, 6, 123-126. Maksimovit, Z. (1966) ,β-kerolite-pimelite series from Goleg Mountain, Yugoslavia. Proc. Internat. Clay Conf. 1966, Jerusalem, I, 97-105. Maksimovit, Z. (1973) Lizardite-nepouite isomorphic series. Zap. Vses. Mineral. Obshch., 102, 143-149. Orcel, J., Hénin, S., and Callère, S. (1949) Sur les silicates phylliteux des minéraux de fer oolithiques. C. R. Acad. Sci. Paris, 229, 134-135. Pattiaratchi, D. B., Saari, E., and Sahama, Th. G. (1967) Anandite, a new barium-iron silicate from Wilagedera, North Western Province, Ceylon. Mineral. Mag., 36, 1-4. Peacor, D. R., Essene, E. J., Simmons, W. B., Jr., and Bigelow, W. C. (1974) Kellyite, a new Mn-Al member of the serpentine group from Bald Knob, North Carolina, and new data on grovesite. Am. Mineral., 59, 1153-1156. Pedro, G. (1967) Commentaires sur la classification et la nomenclature des minéraux argileux. Bull. Groupe fr. Argiles, 19, 6986. Perrault, G., Harvey, Y. and Pertsowsky, R. (1975) La yofortierite, un nouveau silicate hydraté de manganèse de St.-Hilaire, P. Q. Can. Mineral., 13, 68-74. Schaller, W. T. (1930) Adjectival ending of chemical elements used as modifiers to mineral names. Am. Mineral., 15, 566-574. Schaller, W. T., Carron, M. K., and Fleischer, M. (1967) Ephesite, Na(LiAl2)(Al2Si2)O10(OH)2, a trioctahedral member of the margarite group, and related brittle micas. Am. Mineral., 52, 16891696. Semenov, E. I. (1969) Mineralogy of the Ilimaussaq Alkaline Massif Southern Greenland. (in Russian) Inst. Mineral., Geokhim., Kristallokhim. Redk. Elementov, Izdat. "Nauka" 1969. Shimoda, S. (1969) New data for tosudite. Clays & Clay Minerals, 17, 179-184. Springer, G. (1976) Falcondoite, nickel analogue of sepiolite. Can. Mineral., 14, 407-409. Tien, P-L., Leavens, P. B., and Nelen, J. A. (1975) Swinefordite, a dioctahedral-trioctahedral Li-rich member of the smectite group from Kings Mountain, North Carolina. Am. Mineral., 60, 540-547. Veniale, F. and van der Marel, H. W. (1969) Identification of some 1:1 regular interstratified trioctahedral clay minerals. Proc. Internat. Clay Conf. 1969, Tokyo, 1, 233-244. Wada, K. and Yoshinaga, N. (1969) The structure of "imogolite". Am. Mineral., 54, 50-71. Yoshii, M., Maeda, K., Kato, T., Watanabe, S. Yu., Kato, A., and Nagashima, K. (1973) Kinoshitalite, a new mineral from the Noda-Tamagawa mine, Iwate Prefecture. (in Japanese) Chigaku Kenkyu, 24, 181-190. Zhoukhlistov, A. P. and Zvyagin, B. B. (1977) Determination of the crystal structure of chapmanite and bismutoferrite by high-voltage electron diffraction. Sov. Phys.-Crystallogr., 22, 419-423 (Engl. transl.). Zhoukhlistov, A. P., Zvyagin, B. B., Soboleva, S. V., and Shlain, L. B. (1974) The crystal structure determination of chapmanite SbFe2Si2O8(OH) by high-voltage electron diffraction. Coll. Abstr. I.M.A. General Meeting, Regensburg, 79. Manuscript received, August 13, 1979; NOTE 1 for the AIPEA Nomenclature Committee: A. Alietti (Italy), G. W. Brindley (U.S.A.), M. L. L. Formosa (Brazil), K. Jasmund (F. R. Germany), J. Konta (Czechoslovakia), R. C. Mackenzie (U.K.), K. Nagasawa (Japan), R. A. Rausell-Colom (Spain), and B. B. Zvyagin (U.S.S.R.). *The correct adjectival modifier is nickeloan. [var:'startyear'='1980'] [Include:'footer.htm'] |