|
|
Volume 77, pages 1266-1274, 1992 The light-induced alteration of realgar to pararealgar
D. L. DOUGLASS, CHICHANG SHING
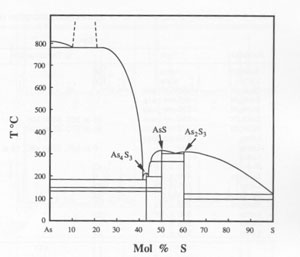
GE WANG ABSTRACT Realgar from various locations, high-purity synthetic realgar, and synthetic realgar with 2 mol% Sb were subjected to unfiltered sunlight, filtered sunlight, and filtered light from a quartz-tungsten-halogen lamp for various times. Both single-crystal and powder samples were used. All of the realgar transformed to pararealgar at wavelengths between about 500 and 670 nm, but no transformation occurred at wavelengths greater than about 670 nm. The reaction rate decreased at wavelengths above 560 nm and was very slow at wavelengths greater than 610 nm. The high-temperature polymorph, ß, also transformed to pararealgar. The alteration to pararealgar did not proceed directly; an intermediate phase, χ, of unknown crystal structure but having the same stoichiometry as realgar, formed first. The time to form χ increased with increasing wavelength at a constant flux of photons. There was no measurable difference in stoichiometry between realgar and pararealgar; both were slightly hyperstoichiometric. Pararealgar forms on the surface as a thin layer or nodules and then fissures at some critical thickness, causing the well-known degradation of realgar exposed to light. The reaction is reversible at elevated temperatures. In all cases, even below the α-ß realgar transformation temperature, χ always formed initially, followed by the formation of β. The a phase then formed from the ß phase at a rate dependent upon temperature. The time to transform ß (formed during the reverse reaction) was about 1 d at 220 °C and 2 d at 175 °C, whereas transformation of normal ß, cooled from above the transformation temperature to either 175 or 220 °C, takes several months. It is proposed that light breaks As-As bonds, which are weaker than As-S bonds, and that the covalently bonded cage molecules form a new crystal structure in which free As is intercalated. The behavior is compared to the photo-decomposition of orpiment, which has been studied extensively. INTRODUCTION The alteration of realgar (AsS) to a friable yellow-orange powder is well known and is a phenomenon that has caused chagrin among most mineral collectors and museum curators. It is stated in most handbooks and mineralogy books that the alteration products are orpiment (As 2S3) and arsenolite (As2O3) (Battey, 1972; Sinkankas, 1966; Vanders and Kerr, 1967; Mottana et al., 1978). The formation of As2S3 in ambient air is not possible, however, because this compound is less stable thermodynamically than realgar and thus has a higher equilibrium dissociation pressure of S. The partial pressure of S, even in industrial environments, is much less than that required to form orpiment.Much has been reported on the crystal structure of realgar, the phase transformation, and stability of the polymorphs (Clark, 1970; Ito et al., 1952; Lu and Donohue, 1944; Mullen and Nowacki, 1972; Porter and Sheldrick 1972; Roberts et al., 1980; Roland, 1972; Street and Munir, 1970), but there remains much confusion in the literature. However, some significant observations made by Cahoon (1965) showed that a new phase formed during the alteration of realgar from Pampa Larga, Chile. Hall (1966) identified the alteration product as -γ-AsS, which formed by exposure to infrared radiation in a vacuum below 130 °C. The γ-AsS formed from both natural and synthetic realgar. Roberts et al. (1980) identified a new polymorph of AsS, pararealgar, from two locations in British Columbia, Mount Washington on Vancouver Island and the Gray Rock property, Lillooet district. Roberts et al. pointed out that pararealgar was the alteration product of realgar and that orpiment was not produced. It is most likely that Hall's γ-AsS is actually pararealgar. Pararealgar appears to be an equilibrium phase in the presence of light, but it is not included in the phase diagram determined by Blachnik et al. (1980). The diagram in Figure 1 shows the existence of three sulfides, As 4S3, AsS, and As2S3, all of which melt congruently. Eutectics occur between As4S3 and As and between AsS and realgar.Likewise, a eutectic exists between realgar and orpiment. No range of composition is given for realgar, which is shown as a line compound on the diagram. The transformation temperature is given as 255 °C, although Roland (1972) reported that the temperature was composition dependent, i.e., 239 °C in the presence of As, 252 °C for stoichiometric AsS, and 263 °C in the presence of As2S3 and S vapor. These results led Roland to suggest that there might be a slight range of stoichiometry of realgar which he stated is "certainly much less than 5%." A spectacular specimen of realgar (crystals about 5-10 mm in size) on a gray quartz and white calcite matrix, from the Getchell mine, Nevada, in the collection of one of the authors (D.L.D.) altered significantly in artificial room light with some direct sunlight (about 1 h/d in a few months). X-ray diffraction of the yellow-orange alteration product clearly showed that pararealgar had formed. The apparent light-induced transformation, the fact that pararealgar was not shown as an equilibrium phase on the phase diagram, and the fact that not all realgar samples are prone to alteration (Clark, 1970) prompted research concerning the transformation in an effort to understand the process. Prior to presentation of results obtained in this study, it is worthwhile to discuss some previous work on crystal structures and to point out some of the inconsistencies among the various studies. BACKGROUND The low-temperature structure is usually designated as realgar (or ß-AsS) and has a monoclinic structure (Ito et al., 1952), with a = 9.27, b = 13.50, c = 6.56 Å, and ß = 106°37'. Mullen and Nowacki (1972) also found a monoclinic structure with a = 9.325, b = 13.571, c = 6.587 Å, and ß = 106°24', values very similar to those of Ito et al. The high-temperature form is usually designated as α-AsS, although Street and Munir (1970) used α-AsS for the low-temperature structure and ß-AsS for the high-temperature form. Porter and Sheldrick (1972) also used the terminology of Street and Munir. Care must be taken to ascertain whether a given reference used α-AsS for the high-temperature or for the low-temperature modification; much confusion exists over terminology. In discussing the experimental results of the present work, we will refer to the low-temperature form as α and the high-temperature form as ß. The high-temperature structure was reported by Porter and Sheldrick (1972) to be monoclinic with a = 9.957, b = 9.335, c = 8.889 Å, and ß = 102°29'. Roland (1972) prepared the high-temperature phase by reacting As 4S3 with S vapor using materials of 99.999% purity. Roland also found a monoclinic structure with a = 9.92, b = 9.48, c = 8.91 Å, and ß = 101°50'. Street and Munir (1970) synthesized AsS from material of 99.9999% purity and determined the high-temperature phase to be orthorhombic, with a = 9.95, b = 13.49, and c = 7.07 Å. Their low-temperature form was found to be monoclinic with lattice parameters virtually identical to those of natural realgar from Humboldt County in Nevada. Kutoglu (1976) reacted pure S and As at 500-600 °C and quickly cooled the product to room temperature. The orange-yellow phase was monoclinic with a = 11.193, b = 9.994, c = 7.153 Å, and ß = 92°48'.
There seems to be good agreement for the low-temperature phase with respect to its structure (monoclinic), lattice parameters, and the value of ß. However, this is not the case for the high-temperature phase. Porter and Sheldrick (1972) and Roland (1972) reported that the high-temperature phase is monoclinic. The lattice parameters and values of ß determined by these researchers are reasonably similar. However, Street and Munir (1970) found an orthorhombic structure, and Kutoglu (1976) found a monoclinic structure whose parameters differed considerably from those of Porter and Sheldrick (1972) and Roland (1972). It should be noted, however, that Street and Munir's d values and intensities are very similar to those of both Clark (1970), see below, and Roland (1972), even though their structure analysis gave different results. Roberts et al. (1980) determined that the structure of pararealgar is monoclinic, with a = 9.929, b = 9.691, c = 8.503 Å, and ß = 97°4'. It is interesting to note that the new polymorph, pararealgar, has a structure whose lattice parameters are fairly close to those of the high-temperature polymorph. Clark (1970) studied realgar from Mina Alacrán, Pampa Larga, Chile, and noted that specimens which had been exposed to sunlight for at least 20 yr, as well as specimens removed directly from the underground mine (no light), had identical X-ray patterns, and there was no evidence of alteration on the samples that had been exposed to sunlight. The patterns were identical to those of Hall (1966) for synthetic α-AsS, the low-temperature form. No changes were observed in Clark's samples after grinding under toluene and subsequent exposure to sunlight for over 2 yr. Exposure to infrared radiation also failed to cause alteration. Samples examined from the Mina Alacrán dump showed no alteration from long-time exposure to light. Surprisingly, material removed from the centers of those samples gave X-ray patterns of the high-temperature polymorph, the patterns being identical to those previously given by Cahoon (1965), Hall (1966), and Roland (1972). Sunlight exposure of these samples for 2 yr failed to cause alteration. Although Cahoon (1965) reportedly observed alteration of realgar from Pampa Larga in Chile, Clark's work suggests that the presence of the high-temperature phase may have a relationship to the alteration process. As stated earlier, a comparison of the lattice parameters and so forth of the high-temperature form as determined by Porter and Sheldrick (1972) and by Roland (1972) to the new polymorph, pararealgar, shows that very little difference exists between the two structures.
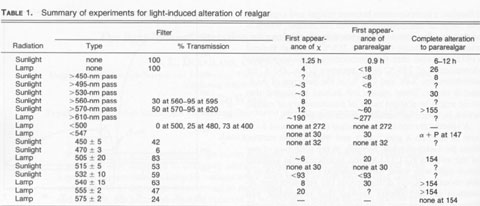
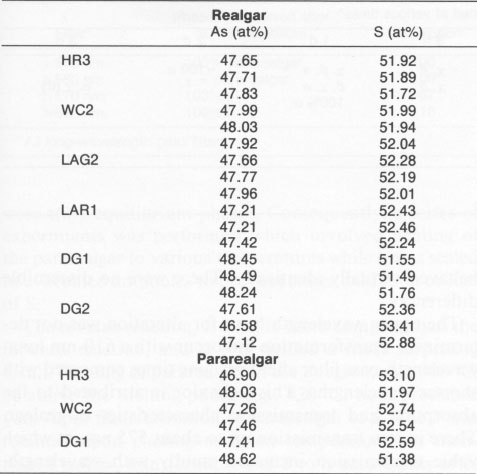
EXPERIMENTAL PROCEDURES Both natural realgar and synthetic compounds were utilized. The natural realgar was in the form of irregular pieces about 0.5-1 cm in size. These included material from the Getchell mine in Nevada, supplied by Anthony R. Kampf, Natural History Museum of Los Angeles County; from Sacramb (Nagyag), Romania, supplied by Carl A. Francis, Harvard University Mineralogical Museum (catalog no. 131525); and from Hunan Province, China, supplied by Chris Wright, Hot Springs, Arkansas. The natural realgar samples were mounted in lucite and ground and polished to give a flat, mirror-like finish. The synthetic samples were prepared from As and S of 99.9999% purity. Stoichiometric amounts of each element were weighed and placed in a vitreous silica ampoule. The ampoule was evacuated and sealed under vacuum. The ampoules were placed in a furnace held at 280 °C (above the transformation temperature but below the melting point) and at 240 °C (just below the transformation temperature). The samples were maintained at the desired temperatures for 24 hr, cooled to room temperature, and subjected to X-ray diffraction (XRD), using a G.E. diffractometer with filtered CuKα radiation. Other ampoules were heated above the melting point and then cooled. In all cases, the high-temperature structure (designated as ß) was retained at room temperature. Transformation of the ß phase to the low-temperature a phase occurred after annealing for several weeks at 240 °C. The high-temperature phase was remarkably stable at room temperature, as previously noted by Street and Munir (1970). The synthetic realgar formed as rather small particles; thus they were ground further prior to investigation. All of the synthetic materials were tested as powders. The bulk natural samples were exposed to light in their mounts. The powder samples of either synthetic material or of pulverized natural crystals were placed on a double-sided cellophane tape and attached to a plastic plate for light exposure and subsequent X-ray diffraction. The bulk samples were used for the observation of a given area in the SEM, after light exposure for various periods, to follow the transformation microscopically. Realgar from all sources was exposed initially to unfiltered sunlight. In order to determine if the alteration was enhanced by various wavelengths, a series of filters was used. A UV-IR pass filter was used initially. This filter blocked out all of the visible spectrum and passed both UV and IR. No transformation occurred after two weeks. This result clearly showed that wavelengths in the visible spectrum were required. Accordingly, long-wavelength-pass filters of 455, 495, 510, 530, 560, 570, and 610 nm were used. In addition, two short-wavelength-pass filters of 500 and 547 nm were used. Several narrow-band-pass filters in the range of interest were employed also. Their characteristics are described with the results of these experiments in Table 1. It was of considerable interest to determine if realgar is stoichiometric or if it exhibits a range of composition, and if a range exists, if it is related to the transformation. High-purity materials were used to prepare As4S3, AsS, and As2S3. An evacuated capsule was prepared in which As4S3 was placed at one end and AsS placed at the other end. A similar experiment was performed with AsS and As2S3. Long-time experiments of two months were performed at 240 °C so that equilibrium could be attained. If AsS exists over a range of composition, the capsule with As4S3 should produce AsS 1-x, and the capsule with As2S3 should produce AsS1+x. After exposure the samples were analyzed by XRD and electron microprobe analysis (EMPA) using a Cameca Camebax electron microprobe operated at 15 kV and 12 mA, with pure As and pure S as standards. Natural realgar and pararealgar produced by the transformation were analyzed also by EMPA and XRD.TABLE 2. XRD data for the χ phase
Photomicrographs were made in a Cambridge Stereoscan scanning electron microscope (SEM). It was possible to obtain good images in some cases without coating the samples. In other cases, the samples charged, and poor images were obtained. It was decided not to coat the samples with C during the experiment, because the coating would absorb visible radiation. Coating was performed only after an experiment had been finished. Optical transmission was measured with an HP 8452A diode array spectrophotometer. Measurements were made on single crystals that had been mounted in resin and polished. The polished mount was sliced to produce a wafer 0.1 cm thick. The cut side was polished, and the samples were then subjected to measurement. The resistance of the Alacrán (Chile) realgar to alteration, (Clark, 1970), raised the question of whether the presence of the high-temperature ß phase in that material might have had an influence. Also, the fact that Sb is commonly detected in most realgars suggested that perhaps impurities might affect the alteration. Synthetic realgars were prepared with pure As, S, and Sb (99.9999% purity). AsS and AsS containing 2 mol% Sb were prepared by melting in evacuated quartz ampoules. RESULTS Wavelength effects All realgar altered to pararealgar in unfiltered sunlight. XRD patterns show peaks for both realgar (designated as α hereafter) and pararealgar (P). No reaction occurred during exposure for at least one month in the laboratory under fluorescent lighting.
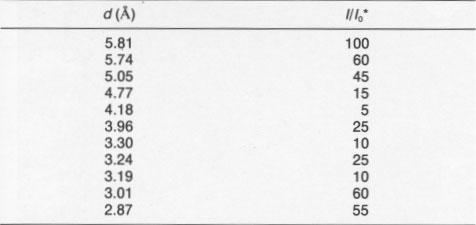
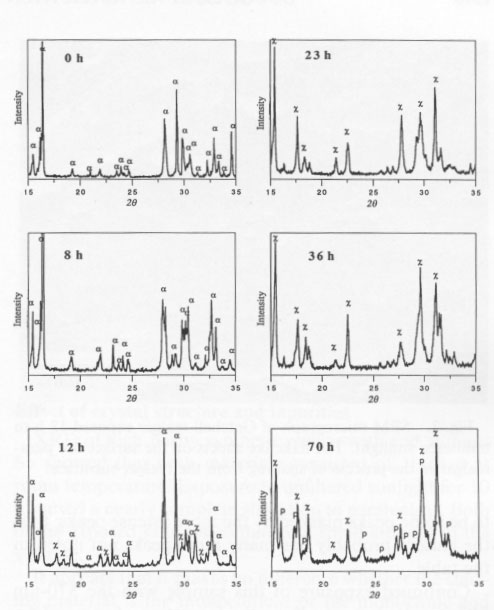
It was of interest to determine if ultraviolet (UV), visible, or infrared (IR) radiation caused the alteration. Accordingly, a filter was used that passed both UV and IR but screened the visible radiation. Single-crystal samples of Getchell realgar were exposed for 85 h. XRD showed only a peaks with no evidence of alteration. Once both UV and IR were ruled out, a series of long-wavelength-pass filters was used. No alteration was observed by exposure for 70 h to light having wavelengths greater than 610 nm. A 570-nm-pass filter was used next, the results of this test giving a most unexpected result. Starting at 2 h exposure, some low-intensity XRD peaks were observed that could not be indexed as α, ß, or P. The unknown peaks increased in intensity with increasing exposure time, whereas the α peaks decreased in intensity. Both the unknown peaks, corresponding to an unknown phase (hereafter referred to as χ), and α peaks coexisted through 36 h exposure. At 58 h, 11 peaks existed, all of which were unidentifiable. The change in the XRD patterns as a function of exposure time is shown in Figure 2. XRD data for the χ phase are summarized in Table 2. The XRD pattern for this phase is typical of that of a layered structure, as noted by line broadening and asymmetrical peaks (Wagner, personal communication, 1991). There appear to be some peaks masked by the more intense peaks; thus the pattern probably has many more peaks not noted in the table. Continued exposure of this sample with the 570-nm filter resulted eventually in the formation of pararealgar, which coexisted with the χ phase at 78 h. A further increase in the exposure showed that the intensity of the χ peaks decreased, whereas the intensity of the pararealgar peaks increased, showing that pararealgar formed at the expense of the χ phase. A similar set of experiments to the above was performed with the 560-nm filter. No changes were detected during 4-h exposure. A small amount of χ was detected at 8 h, and at 16 h the sample had been completely transformed to χ. Pararealgar appeared after 20 h, and both χ and pararealgar existed at 30 h. The behavior of realgar exposed under the 560-nm filter is basically the same as that exposed to the 570-nm filter. However, there was a marked difference in the rate of alteration. Complete alteration occurred in 8 h with a 455-nm filter and in 30 h with a 530-nm filter, both the χ phase and pararealgar coexisted at 70 h with a 560-nm filter, only the χ phase formed during 70 h with a 570-nm filter, and, finally, no alteration at all was observed after 70-h exposure with a 610-nm filter.
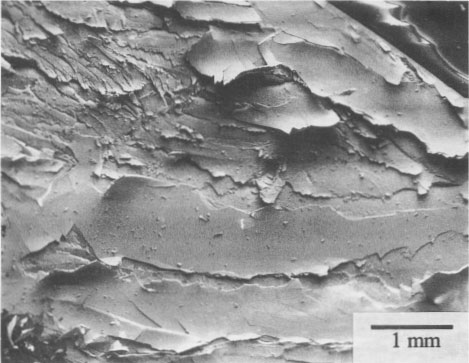
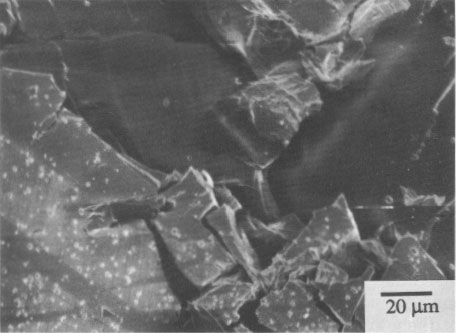
SEM micrographs of alterations Pararealgar appears to form on the realgar surface as a thin layer that initially adheres to the surface. It is not known if the two phases are epitaxially related. The pararealgar is apparently less dense (more voluminous) than a realgar, and eventually a limiting thickness is reached at which the adherent pararealgar fissures and spalls. An example of this behavior is shown in Figure 3 of a single crystal of Getchell realgar. The χ phase is seen in Figure 4 on a single crystal of Romanian realgar (HR2), which was exposed for 58 h with the 570-nm filter. The pararealgar is the light phase that charged in the SEM. This is indicative of a lower electrical conductivity than that of either realgar or of the χ phase.
TABLE 3. Electron microprobe analyses
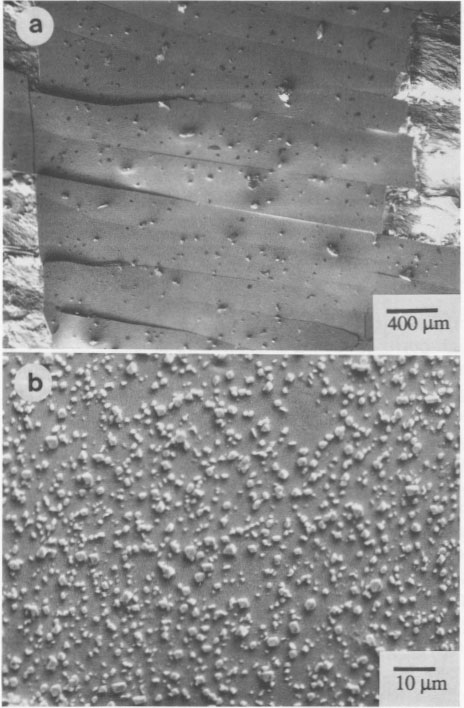
Another single crystal, Chinese realgar (WC 1), formed a sheet of the χ phase that partially spalled and that exhibited a series of parallel fractures, as noted in Figure 5a. A higher magnification of the sheets (Fig. 5b) shows many small particles, which are pararealgar. Pararealgar nucleates on the χ phase, which is a precursor that appears to be necessary for pararealgar formation. Compositional variations EMPA was used to determine the composition and to discern if nonstoichiometry existed. High-purity As and high-purity FeS, were used as standards. Three areas were analyzed from numerous crystals. The S content was found to always be greater than 50 at%. The accuracy of the measurements is ±0.5%, and, since the S content was approximately 52 at%, it was concluded that a realgar is indeed S rich. Data are summarized in Table 3. The composition of pararealgar could not be determined accurately by wavelength-dispersive (WDX) EPMA. This is due to the flocculent, porous mature of this phase. Accurate EPMA requires a fully dense phase with a planar surface. EDX was therefore employed, although it may be inherently less accurate than WDX. Nevertheless, the crystals previously analyzed by WDX were used as standards for EDX. Within the accuracy of the technique there was no compositional difference between realgar and pararealgar. They are both slightly hyperstoichiometric. (Hyperstoichiometry cam result from either As vacancies or interstitial S.)
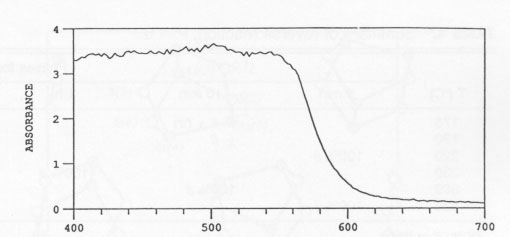
Effect of crystal structure and impurities XRD of high-purity synthetic realgar with and without Sb showed that both materials consisted of 100% ß at room temperature. Exposure to unfiltered sunlight for 10 h caused a nearly complete alteration to pararealgar. Both of the exposed samples contained a small amount of the χ phase. It appears that it makes no difference whether the starting material is the monoclinic α or the monoclinic and orthorhombic β phase. The behavior was identical. It also appeared that the presence of Sb had no effect. It should be noted that the Sb was in solid solution, since no evidence of Sb2S3 was detected. EDX analysis of the realgar containing Sb readily detected the Sb. Optical transmission A typical absorbance spectrum of a single crystal of a realgar from Romania, which was about 0.1 cm in thickness, is shown in Figure 6. The absorbance was essentially 100% at wavelengths below 565 mm and decreased rapidly with increasing wavelength above this value, showing a sigmoidal curve. Reversibility of the transformation Samples of 100% pararealgar formed by alteration of pulverized natural realgar from all three sites (China, Romaina, and Getchell), were sealed in quartz ampoules under vacuum and heated at various temperatures. Samples heated to 300 °C, in the ß-phase field, transformed to ß in 5 min. Samples held at 420 °C, above the melting point of realgar, also formed ß in 5 min. In every case the color changed from yellow-orange to bright red. Experiments performed at various temperatures within the a-phase region yielded some unexpected results. First, heating to 220 °C produced ß in 5 min even though the lowest reported α-ß transformation temperature is 239 °C. A Getchell sample was held for two months at 220 °C and was transformed to equilibrium α phase during this period. Treatment at 175 °C showed no reaction in 5 min, but the unknown χ phase formed in 50 min. Longer times at 175 °C produced ß. Reaction at 190 °C for 1 d resulted in a mixture of all four phases, P, χ, ß, and α. A summary of results obtained during the reverse reaction is given in Table 4. TABLE 4.
Summary of reverse reaction, P → α
Note: tr = trace.
Irradiation using a Tungsten-quartz-halogen lamp The vagaries of weather prompted the use of artificial light. This provided a constant source of irradiation 24 h/d as opposed to the variations in sunlight intensity with time of the day, day of the year, partial cloud cover, etc. The lamp used was a QL-500-WL manufactured by the Regent Lighting Corporation. A photocell indicated that the power density at the sample surface was 53 mw/cm2 at a distance of 30 cm (about 65-70% of natural sunlight) and 28 mw/cm2 at a distance of 45 cm. Initial experiments were performed with the light at a distance of 30 cm from the samples. Temperature measurements showed that the sample temperature reached 60 °C. The somewhat elevated temperature eventually led to some As 2O3 formation after long times. Complete oxidation occurred in 1200 h. In order to reduce the temperature, the samples were placed 45 cm from the light. The temperature reached 40 °C.Results obtained were basically the same as those obtained in sunlight. The times required for initiation and completion of alteration were slightly longer with the lamp than with sunlight because of the reduced intensity of radiation. The most significant observation was that longtime exposures with the 610-nm long-wavelength-pass filter caused alteration, and that the 610-nm wavelength was not a cutoff value below which alteration occurred and above which it did not. For example, χ and a coexisted after 190 h, and pararealgar started to form after 277 h. DISCUSSION The alteration of realgar to pararealgar is dependent on the wavelength of the light to which the realgar is exposed, as determined from a number of experiments employing various types of filters. It appears that no alteration occurs at wavelengths shorter than about 500 nm. The exact wavelength value below which there is no reaction is difficult to determine because filters do not have step-function characteristics. It is clear, however, that visible light is required, because no alteration was observed with a filter that passed both UV and IR radiation but blocked out all of the visible. Realgar from all sources behaved virtually identically. There were no discernible differences. The upper wavelength limit for alteration was not determined. Transformation did occur with a 610-nm long-wavelength-pass filter after very long times compared with shorter wavelengths. This behavior is attributed to the absorption and transmission characteristics of realgar. There is zero transmission up to about 575 nm, at which value transmission increases rapidly with wavelength, following a sigmoidal relationship. This means that wavelengths greater than 610 nm are basically passing through the crystal without being absorbed, and little energy is being transferred to break bonds. The fact that alteration takes so much longer with longer wavelengths, which are mostly transmitted through the crystal, strongly suggests that a certain number of photons of light is necessary to cause the reaction to take place. Another experiment that supports this hypothesis is the one in which a narrow-band filter of 575 nm was used. This filter was very sharp, passing 575 ± 2 nm with a total transmission of only 24%. Thus, the number of photons passing through this filter was extremely small, and no alteration was observed after 154 h. A kinetics effect (described below) was clearly apparent in the range of 560-610 nm. Long-wavelength-pass filters of 530, 560, 570, and 610 nm (Table 5) showed that for a given time, 30 min, complete alteration to pararealgar occurred with the 530-nm filter, but no alteration had been observed with the 610-nm filter. Since the filters pass all wavelengths greater than the indicated value, there is apparently a higher efficiency of light in the range of 530-560 nm for alteration than there is above 560 nm. All wavelengths are absorbed in the range of 530-560 nm; thus the energy of the incident light is important in addition to the number of photons absorbed. The alteration process involved formation of an intermediate phase, χ, for which the X-ray diffraction pattern could not be identified in the JCPDS file. The phase seemed to be the precursor to pararealgar, but its presence was difficult to detect in experiments involving shorter wavelengths of 500-530 nm because the reaction was so much faster than at wavelengths greater than 560 nm. The unknown phase appears to have a layered type of structure based on the shape of peaks in the X-ray pattern. It was not known initially if either χ or pararealgar were truly equilibrium phases. Consequently, a series of experiments was performed which involved heating of the pararealgar to various temperatures while it was sealed in evacuated ampoules to prevent either oxidation or loss of S.
TABLE 5. Effect of wavelength on realgar alteration
The reverse reaction, P → α, is more complex than the forward reaction, and it too yielded a most surprising result. Heating of pararealgar in the ß region, e.g., 300 °C, or above the melting point, e.g., 420 °C, both produced very rapid transformation of pararealgar to ß. The ß was retained upon cooling to room temperature. As mentioned earlier, transformation of ß to α occurs only after long times at temperatures close to the transformation temperature, as determined by Roland (1972) and confirmed by us. However, the reverse reaction in the α-phase region resulted in the formation of χ at 175 °C in 50 min and ß at 220 and 230 °C in 5 min. The formation of ß at temperatures markedly below the α-ß transformation temperature is most unusual, but it may be related to the χ phase, which appears to be an intermediate precursor between pararealgar and ß during the reverse transformation. The ß formed during the reverse reaction at 220 °C transformed to a after only 1 d, compared with a time of several weeks for normal ß (ß not involved in the reverse reaction). Obviously, the temperature of the furnace was well below the α-ß transformation temperature, and the fact that ß formed prior to α cannot be attributed to an extraneously high temperature. The above experiments strongly suggest that both χ and pararealgar are equilibrium phases. Transition phases occur commonly in many solid-state transformations; a classical example is the age hardening of Al-Cu alloys (Shewmon, 1969). Quenching of the high-temperature solid solution (supersaturated α) to an intermediate temperature below the solvus causes formation of Guinier-Preston zones, followed by formation of θ", θ', and eventually equilibrium θ in a solid solution of α that has the equilibrium composition given by the Al-Cu phase diagram. Reheating does not involve any of the transition phases. They are metastable, and their formation occurs because each one provides a small decrease in free energy, eventually leading to equilibrium. All realgar that was tested readily altered to pararealgar. The samples included material from the Getchell mine in Nevada, from China, and from Romania. The most difficult thing to explain is why the realgar from Pampa Larga, Chile (Clark, 1970), did not transform after 20 yr of exposure to sunlight in the mine dump, and why material taken from the mine did not alter under direct sunlight. The unique aspect to this realgar was the presence of the high-temperature phase, ß, which is not usually present. Perhaps the closeness of the structures of both pararealgar and ß precludes the formation of pararealgar. It would be of great interest to study this material if some were available.
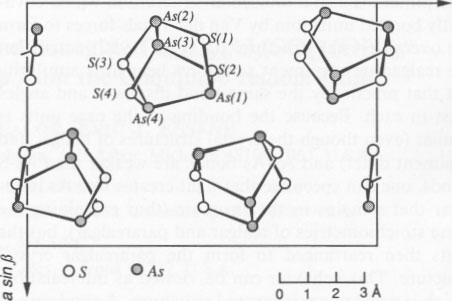
Another perplexing aspect is what the light is actually doing to the realgar structure. Let us examine the structure in more detail. Realgar is most properly represented by the formula As4S4. This is a cage structure (sometimes referred to as a cradle structure) in which As atoms form a tetrahedron (also referred to as a bisphenoid) and a square of S atoms. The cage molecules are covalently bonded. Each As atom is bonded to three nearest neighbors, two S and an As atom. The crystal structures of both α realgar and β realgar form by joining of the tetrahedra by weak Van der Waals forces, as seen in Figure 7, a unit-cell projection on [001] of a (Mullen and Nowacki, 1972). Dimensions of the cage structure (Ito et al., 1952) are as follows: As-S bonds = 2.24 Å, As-As bonds = 2.59 Å, As-S-As angle = 101°5', S-As-S angle = 92°8', and S-As-As angle = 97°8'. Yang et al. (1987) studied the temperature dependence of extended X-ray absorption fine structure spectra and concluded that the As-S bonds are about 30% stronger than the As-As bonds. Keneman et al. (1978) researched the behavior of orpiment and realgar when exposed to light and thermal cycling in order to understand the optical-storage effect of these compounds. They propose that photodecomposition causes the following reaction in orpiment: As2S3 → yAs + As2-yS3, which results in the formation of clusters of free As. The reaction was reversible upon heating. However, their work was on thin vapor-deposited films, and it appears from their remarks that equilibrium did not exist in the films. Unexposed films reportedly consisted of As2S3, As2S2, and S.Orpiment is also a molecular structure in which covalently bonded units join by Van der Waals forces to form the overall crystal structure. Ito et al. (1952) noted that the realgar and orpiment structures bear little similarity but that practically the same bond distances and angles exist in each. Because the bonding of the cage units is similar (even though the crystal structures of realgar and orpiment differ) and As-As bonds are weaker than As-S bonds, one can speculate that light creates free As in realgar that remains in the structure (thus explaining the same stoichiometries of realgar and pararealgar), but the units then rearranged to form the pararealgar crystal structure. This behavior can be viewed as intercalation, which is well known in layered structures. A good case in point (Chen and Douglass, 1990; Wang et al., 1991) involves intercalation of cations such as Ni2+, and Fe2+ into the layered structure of MoS2 during sulfidation of Ni-Mo and Fe-Mo alloys. The intercalated ions fit into octahedral interstices between the covalently bonded sheets of MoS2, which are held together by weak Van der Waals forces. However, Al3+ is much smaller than the site of the octahedral hole, and destabilization of MoS2 occurs, causing the formation of Al 0.5Mo2S4, an inverse spinel. Thus, it is possible that free As forms by interaction of light with realgar, and the free As destabilizes the realgar structure, even though the basic molecular cage units remain unchanged. This process, as in the case of orpiment, is reversible upon heating.ACKNOWLEDGMENTS Although this research was unfunded, there are many who offered generous support. First, thanks are given to those who supplied samples. These people include Carl A. Francis, Mineralogical Museum, Harvard University; Peter C. Keller and Anthony R. Kampf, Natural History Museum of Los Angeles County; and Chris Wright, Wright's Rock Shop, Hot Springs, Arkansas. Second, numerous lengthy telephone conversations were held with a number of individuals early in the program, who provided much useful information on background, etc. These include Peter Keller and Anthony Kampf, Natural History Museum of Los Angeles County; Gail Dunning, Sunnyvale, California; Carl A. Francis, Harvard University; Taras Bryndzia, University of Chicago; Alan Clark, Queen's University, Ontario, Canada; and George Rossman, California Institute of Technology. Finally, the comments from C.N.J. Wagner, UCLA, concerning the X-ray patterns and suggestions from Jeffrey Zinc, UCLA, about photochemistry were most helpful. Figure 1 is reprinted here with the permission of Barth Verlagsgesellschaft; Figure 7 is reprinted with the permission of R. Oldenbourgh Verlag. REFERENCES CITED Battey, M.H. (1972) Mineralogy for students, 323 p. Oliver and Boyd, Edinburgh. Blachnik, R., Hoppe, A., and Wickel, U. (1980) Die Systeme Arsen-Schwefel und Arsen-Selen und die thermodynamischen Daten ihrer Verbindungen. Zeitschrift für anorganische und allgemeine Chemie, 463, 78-90. Cahoon, B.G. (1965) Polymorphism of arsenic sulfide (AsS). U.S. National Technical Information Service, Document AD 633 148. Chen, M.F., and Douglass, D.L. (1990) Effect of some ternary additions on the sulfidation of Ni-Mo alloys. Oxidation of Metals, 33, 103-133. Clark, A.H. (1970) Alpha-arsenic sulfide from Mina Alacrán, Pampa Larga, Chile. American Mineralogist, 55, 1338-1344. Hall, H.T. (1966) The systems Ag-Sb-S, Ag-As-S, and Ag-Bi-S: Phase relations and mineralogical significance. Ph.D. thesis, Brown University, Providence, Rhode Island. Ito, T., Morimoto, N., and Sandanaga, R. (1952) The crystal structure of realgar. Acta Crystallographica, 5, 775-782. Keneman, S.A., Bordogna, J., and Zemel, J.N. (1978) Evaporated films of arsenic trisulfide: Physical model of effects of light exposure and heat cycling. Journal of Applied Physics, 49, 4663-4673. Kutoglu, V.A. (1976) Darstellung und Kristallstruktur einer neuen isomeren Form von As4S4. Zeitschrift für anorganische und allgemeine Chemie, 419, 176-184. Lu, C.-S., and Donohue, J. (1944) An electron diffraction investigation of sulfur nitride, arsenic disulfide (realgar), arsenic trisulfide (orpiment), and sulfur. Journal of the American Chemical Society, 66, 818-827. Mottana, A., Crespi, R., and Liborio, G., (1978) Guide to rocks and minerals, 607 p. Simon and Schuster, New York. Mullen, P. J., and Nowacki, W. (1972) Refinement of the crystal structures of realgar, AsS and orpiment, As2S 3. Zeitschrift für Kristallographie, 136, 48-65.Porter, E.J., and Sheldrick, G.M. (1972) Crystal structure of a new crystalline modification of tetra-arsenic tetrasulphide (2,4,6,8-tetrathia1,3,5,7-tetra-arsa-tricylco[3,3,0,0]-octane), Journal of the American Chemical Society, 13, 1347-1349. Roberts, A.C., Ansell, H.G., and Bonardi, M. (1980) Pararealgar, a new polymorph of AsS, from British Columbia. Canadian Mineralogist, 18, 525-527. Roland G. W. (1972) Concerning the α-AsS → realgar inversion. Canadian Mineralogist, 11, 520-525. Shewmon, P. (1969) Transformations in metals, 304 p. McGraw-Hill, New York. Sinkankas, J. (1966) Mineralogy, 578 p. Van Nostrand, Princeton, New Jersey. Street, G.B., and Munir, Z. (1970) The structure and thermal properties of synthetic realgar (As4S4). Journal of Inorganic Nuclear Chemistry, 32,3764-3774. Vanders, I., and Kerr, P. (1967) Mineral recognition, 316 p. Wiley, New York. Wang, G., Douglass, D.L., and Gesmundo, F. (1991) High-temperature sulfidation of Fe-30Mo alloys containing ternary additions of Al. Oxidation of Metals, 35, 349-374. Yang, C.Y., Paesler, M.A., and Sayers, D.E. (1987) Determination of bond strengths of arsenic and arsenic chalcogen compounds using the temperature dependence of extended X-ray absorption fine structure. Physical Review B, 36, 980-988.
MANUSCRIPT RECEIVED OCTOBER 15, 1991 [var:'startyear'='1992'] [Include:'footer.htm'] |