
Short Course Notes, Sulfide Mineralogy pages CS21-CS29, 1974
THE Fe-S SYSTEM ( S. D. Scott)
Besides containing two of the most common sulfide minerals, pyrite and pyrrhotite, the Fe-S system is a cornerstone to the understanding of phase relations and thermochemistry of many other important systems including Zn-Fe-S, Cu-Fe-S, Fe-Ni-S and Fe-As-S. The many complexities of the system, some of which are yet unresolved, make it a good place to begin our systematic review of sulfide phase relations.
The basic phase diagram above 400°C is shown in Fig. CS-1. Details at high temperature (>700°C) are in Fig. CS-2 and at low temperatures (<350°C) in Fig. CS-3. Note that the compositions in Fig. CS-1 and CS-2 are in weight percent whereas Fig. CS-3 is in atomic percent Fe. We will focus our attention between the compositional limits FeS to FeS2 by first describing the stoichiometry and stability of the phases in the condensed system followed by discussions of thermodynamics and the effect of pressure on phase relations. The properties of solid phases encountered in the system are summarized in Table CS-1.
Throughout the discussion, superlattice dimensions a and c of pyrrhotites and related phases are presented in terms of A and C, the dimensions of the simple hexagonal subcell with a NiAs (1C) structure.
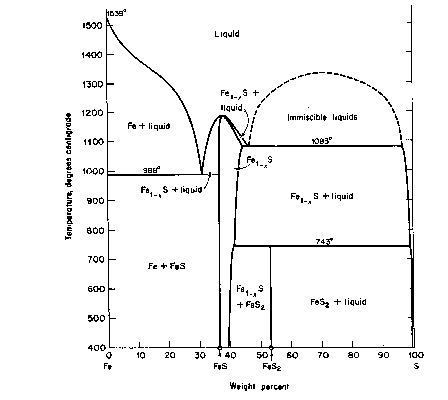
Fig. CS-1. Relations among condensed phases in the system Fe-S above 400°C. (From Ehlers, 1972, after Kullerud, 1967 The Interpretation of Geological Phase Diagrams, Fig. 217, p. 232).
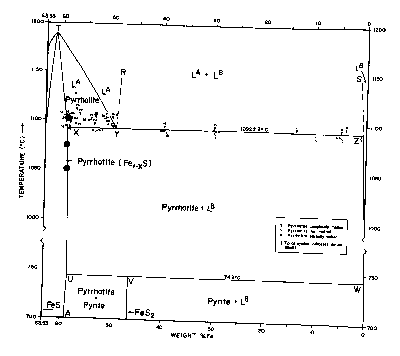
Fig CS-2 Relations among condensed phases in the sulfur-rich portion of the Fe-S system above 700°C. The closed circles are runs which used coexisting sphalerite to determine the phase boundaries as described in the section on Zn-Fe-S. (Modified from Arnold, 1971).
Phases
Troilite (FeS). Strictly speaking, the name troilite applies only to the polymorph of stoichiometric FeS which is stable below 140°C (Gronvold and Haraldsen, 1952; Yund and Hall, 1968). Above 140°C, FeS has the NiAs (1C) structure of high-temperature hexagonal pyrrhotite whose composition range extends for a considerable distance across the system.
Although a common constituent of meteorites, troilite is found only occasionally in terrestrial environments, usually with low-temperature hexagonal pyrrhotite. The solvus separating troilite from other pyrrhotites has been determined most recently by Yund and Hall (1968).
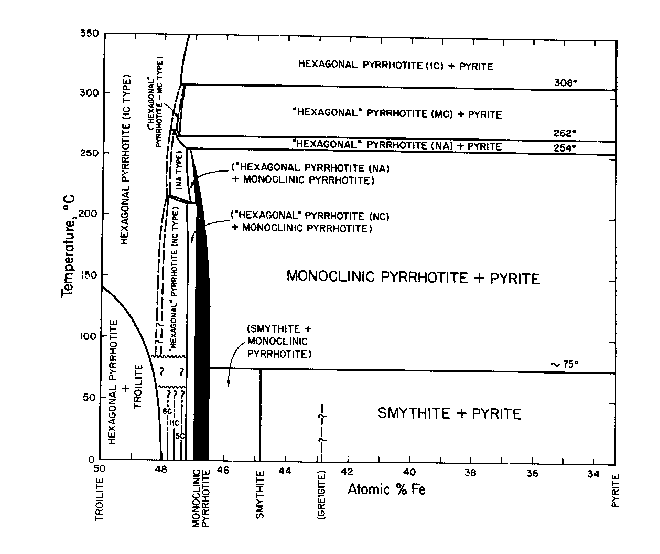
Fig. CS-3. Relations among condensed phases in the central portion of the Fe-S system below 350°C. (Kissin, 1974, modified from Scott and Kissin, 1973).
Mackinawite (FeS1-x). Evans et al (1964) proposed the name mackinawite for a relatively-rare tetragonal mineral of near-FeS composition. It is found either with troilite or with low-temperature pyrrhotites (Kullerud, 1967) in a wide variety of geological environments including: black iron sulfide muds, contact metasomatic and hydrothermal deposits, bedded cupriferous iron sulfide deposits within metamorphic rocks, in certain ultrabasic rocks and in several lunar rocks (Zoka et al, 7.973). Mackinawite invariably contains some Co and Ni although these elements are not essential as demonstrated by Berner (1964), who precipitated the phase from aqueous iron sulfide solutions between 20° and 95°C. The metal to sulfur ratio in mackinawite is slightly greater than unity (1.04 to 1.07; Takeno and Clark, 1967) and the formula is usually written Fe1+xS. However, Taylor and Finger (1971) have shown that there is a deficiency of sulfur in the structure rather than an excess of metal so the formula is properly FeS1-x.
Very little is known about the thermal stability of mackinawite and, for this reason, it is not shown as a stable phase in Fig. CS-1 and CS-3. Zoka et al (1973) found that natural mackinawite from a variety of localities broke down nonisochemically between 120° and 153°C to pyrrhotite of a more S-rich composition. They concluded that their experiments did not represent equilibrium, however, because the expected breakdown assemblage of metallic Fe, Co, Ni + troilite was not found.
"Hexagonal" Pyrrhotite (Fel-xS). There has probably been more written on pyrrhotite than on any other sulfide mineral but, in spite of this voluminous research effort, many enigmas remain, particularly at low temperatures where slow reaction kinetics tend to obscure the phase relations. However, recent studies at low temperatures by Nakazawa and Morimoto (1971) and by Kissin (1974; cf.also Kissin and Scott, 1972; Scott and Kissin, 1973), in which single crystal x-ray diffraction was used as an analytical tool, have reduced what before was chaos to mere confusion.
Between its maximum melting temperature of 1190°C (point T on Fig. CS-2) and 308°C, the full width of the pyrrhotite phase field is occupied by a single solid solution, Fel-xS, in which iron and vacancies are randomly distributed in the cation sites of the NiAs (1C) structure. This phase also extends to lower temperatures but with a more restricted composition range (Fig. CS-3). Disordered 1C pyrrhotite cannot be quenched (Corlett, 1968) and crystals cooled from its phase field acquire one or more of the many superstructures shown on Fig. CS-3. Earlier diagrams which now pervade the literature placed a phase boundary which was independent of composition across the 1C field near 320°C. This beta-transition, as it is known, manifests itself as a discontinuity in magnetic susceptibility attending the antiferromagnetic to paramagnetic Neel transition (van den Berg, 1972). This transition became further entrenched as a phase boundary with the observations of Desborough and Carpenter (1965) that pyrrhotite quenched from above the beta-transition possessed a 2A,7C superlattice whereas those annealed below the beta-transition possessed a 2A,5C superlattice. However, from the work of Corlett (1968), Nakazawa and Morimoto (1971), and Kissin (1974) we now know that the Neel transition does not accompany a phase change. The superstructures observed by Desborough and Carpenter (1965) correlate with some lower temperature phases and undoubtably formed during quenching.
An indication that the simple solid-solution field of hexagonal pyrrhotite would give way to a far more complicated picture at low temperatures was evident from the observations of Carpenter and Desborough (1964), Mukaiyama and Izawa (1966), and Morimoto et a1 (1971) that x-ray diffraction patterns of all natural pyrrhotites contain superlattice reflection at room temperature. Furthermore, Arnold (1967) had shown that although the bulk composition of naturallyoccurring pyrrhotite mixtures spanned nearly the entire range of hexagonal pyrrhotite solid solutions (Fig. CS-4a), the compositional ranges of the constituent pyrrhotite phases were very narrow (Fig. CS-4b), suggesting the interruption of the hightemperature solid solution by at least two solvi.
The overall picture which has emerged from the single crystal x-ray studies of Nakazawa and Morimoto (1971) and Kissin (1974) is one of increased ordering of vacancies spreading across the pyrrhotite phase field with decreasing temperature
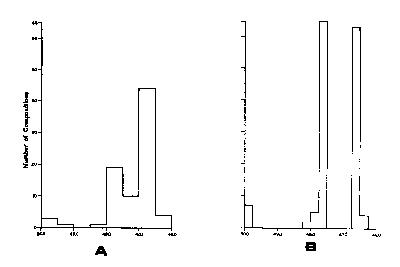
Fig. CS-4. Frequency distribution of natural terrestrial pyrrhotite compositions (From Arnold, 1967). (a) Bulk compositions of pyrrhotites; (b) composition of individual pyrrhotite phases in the bulk. (Composition is in atomic percent Fe.)
(Fig. CS-3) and giving rise to the various superstructures. Monoclinic pyrrhotite (4C) also fits this scheme but is discussed separately below. The 5C, 11C and 6C types found at room temperature are stoichiometric phases with compositions Fen-1Sn (n = 10, 11 and 12, respectively) and are separated by two-phase fields (Morimoto et al, 1971). Between the upper stability limit of the three stoichiometric types (which is unknown but probably below 100°C) and 308°C there are three other phases which also possess sub cells of the NiAs structure but have supercells of uncertain symmetry. These phases are distinguished by their nonintegral repeats of x-ray reflections along hexagonal a* or c* axes of the subcell and have been termed, NA, NC, and MC types of Nakazawa and Morimoto (1971). Their reciprocal a*c* planes are shown in Fig. CS-5. In the NC type the periodicity of the superlattice reflections parallel to c* (Δl) can vary continuously from 1/6 to 1/3 of the repeat of the sub cell reflections giving rise to apparent cell dimensions of a = 2A and c = NC. N varies from 3.0 to 6.0 as a function of temperature and composition and is generally nonintegral. The 2A,5C superstructure of Carpenter and Desborough (1964) is probably equivalent to the NC type (Kissin, 1974). The diffraction pattern of the MC type is similar to that of NC. Its apparent cell dimensions are a = 2A and c = MC, where M varies continuous from 3.0 to 4.0. The NA type is characterized by pairs of supercell reflections of alternating intensity along the a* axis. The spacing between the pairs (Δh) changes continuous with increasing temperature from 1/20 to 1/45 of the repeat of the subcell reflections. The apparent cell dimensions are a = NA and c = 3C, where N is 1/2 Δh and varies continuously from 40 to 90.
A final type, "metastable pyrrhotite," is encountered when iron-rich pyrrhotites are quenched into the two phase field of troilite + pyrrhotite. If exsolution does not occur, a metastable, strained structure results which is similar to the MC structure.
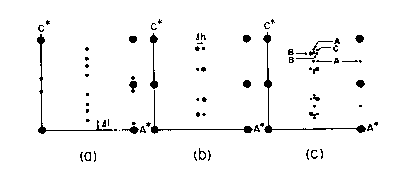
Fig. CS-5. Diagramatic sketches of precession x-ray photographs of the a*c* planes. (a) the NC type; (b) the NA type; (c) the 4C (A), NA (B) and MC (C) types. The large solid circles represent sub cell reflections for the 1C type. (From Nakazawa and Morimoto, 1971 Mater. Res. Bull 6, p. 642).
Monoclinic Pyrrhotite ("Fe7S8"). Ferromagnetic pyrrhotite with a monoclinic superlattice and composition centered about Fe7S8 has been known as a mineral for decades (Bystrom, 1945) and has been synthesized repeatedly in evacuated silica tubes. However, the earlier experimental studies by Clark (1966), Arnold (1969), Yund and Hall (1969) and Taylor(1970a), to name just a few, gave results that were conflicting and were plagued by demonstrable, extensive metastability. Such metastability in evacuates silica tube experiments is not surprising in view of Yund and Hall's (1970) observation that pyrrhotite oversaturated with respect to pyrite by as much as 0.18 at. % Fe did not nucleate pyrite after annealing for more than a year at 325°C. More recently Kissin (1974; see also Kissin and Scott, 1972; Scott and Kissin, 1973) and Rising (1973) have used the method of hydrothermal recrystallization to overcome these kinetic difficulties and establish phase relations for monoclinic pyrrhotite which are internally consistent and devoid of obvious metastabilities (Fig. CS-3). Kissin has synthesized single crystals of monoclinic pyrrhotite in the temperature range of 115-254°C and obtained a close control on the compositional limits of this mineral. As shown in Fig. CS-3, monoclinic pyrrhotite has variable stoichiometry. It is only nominally Fe7S8 and is separated from the NA and NC-type pyrrhotites by narrow solvi. Kissin's upper stability limit of 254°C for monoclinic pyrrhotite going to NA pyrrhotite + pyrite has been reversed. It is in good agreement with the indirect determination at 251±3°C by Rising (1973) but is considerably lower than previous estimates in the range 292°-325°C from the aforementioned evacuated silica tube experiments.
"Anomalous" Pyrrhotite (Fe7S8). Clark (1966) has described a pyrrhotite containing 46.4 atomic % Fe in which the intensity of the (40bar8) x-ray reflection is greater than (408), the reverse of the usual situation with normal monoclinic pyrrhotite (see Fig. S-11B). This "anomalous" pyrrhotite, as it is called, appears to be widespread in low-temperature, sedimentary environments. Unlike normal monoclinic pyrrhotite which is ferromagnetic, it is anti-ferrimagnetic as is hexagonal pyrrhotite. Its stability field has not been ascertained, although Taylor (1971) has shown that one way in which it can form is by oxidation of hexagonal pyrrhotite.
Gamma Iron Sulfide (Fe2S3). This phase has not been encountered in nature but has been precipitated from an aqueous sulfide solution at 60°C by Yamaguchi and Wada (1973). Electron diffraction patterns indicate that it has a spinel structure similar to greigite, the main difference in their patterns being the intensities of some of the reflections. Like greigite it is magnetic, and Yamaguchi and Wada suggest that γ-Fe2S3 bears the same relation to greigite as γ-Fe2O3 (maghemite) does to magnetite.
Nothing is known about the thermal stability of γ-Fe2S3. Yamaguchi and Wads claim, on the basis of published diffraction intensities, that natural greigite is a solid solution of Fe2S3 and Fe3S4.
Smythite (Fe9S11). Smythite was originally described by Erd et al (1957) as having a rhombohedral structure and a Fe3S4 composition which led Kullerud (1967) to believe that it was a polymorph of greigite. However, after an extensive re-examination of naturally occurring smythites, Taylor and Williams (1972) have redefined the mineral as having a pseudorhombohedral structure related to that of monoclinic pyrrhotite and a composition (Fe,Ni)9S11 (~(Fe,Ni)3.25S4). This composition was also found by Nickel (1972) and Bennett et a1 (1972). Thus, smythite is not a polymorph of greigite but may be another ordered pyrrhotite of the Fen-1Sn clan.
Not much is known about the stability of smythite. It occurs as exsolution lamallae in monoclinic pyrrhotite (Bennett et al, 1972) and in geodes which have fluid inclusion filling-temperatures of 25 to 40°C,suggesting that it is a phase stable only at low temperatures. Kissin (1974) did not encounter smythite in his hydrothermal recrystallization experiments above 115°C. Rickard (1968) precipitated smythite from aqueous sulfide solutions but Taylor (1970b) was unable to duplicate the experiment. Taylor (1970a,b) found that smythite began to break down at 210°C in nonequilibrium experiments but concluded that it must be stable below 75° because he was unable to synthesize it at higher temperatures. .........
[var:'startyear'='1974'] [Include:'footer.htm']