|
|
Volume 60, pages 407-412, 1975
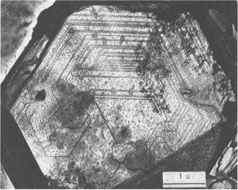
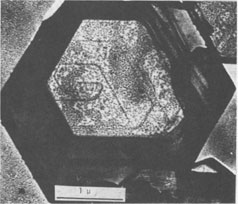
Growth Spirals on Kaolin Group Minerals ICHIRO SUNAGAWA, AND YUTAKA KOSHINO Institute of Mineralogy, Petrology, and Economic Geology, Tohoku University, Aoba, Sendai, 980 JapanAbstract The decoration technique of electron microscopy was applied to kaolinite, dickite, and nacrite from four hydrothermal metasomatic deposits in Japan. Beautiful growth spirals of mono-molecular step heights are observed on their basal planes, showing that they are formed by spiral growth mechanisms from solution phases of low supersaturation. Dickite and nacrite show interlacing patterns, which can be explained by difference in stacking of kaolin layers. Introduction Since Frank (1949) put forward the spiral growth theory to account for the growth mechanism of vapor-phase crystals, a vast amount of evidence in support of the theory has been reported on a wide variety of crystals. Among mineral crystals, growth spirals of mono-molecular step heights have been observed on sphalerite (Verma, 1956; Komatsu and Sunagawa, 1965), biotite (Amelinckx, 1952), hematite (Sunagawa, 1960, 1961, 1962), phlogopite (Sunagawa, 1964), and others. These observations have been made mainly by means of phase contrast and interference contrast microscopy, together with the measurement of the step heights by multiple-beam interferometry. Although these methods are extremely sensitive in vertical resolution, and steps as shallow as a few Ångstroms can be observed (Sunagawa, 1961), the lateral resolution is limited to that of optical microscopy. This is perhaps the reason that only a few examples of growth spirals have been reported on minute crystals such as clay minerals, which are too thick to transmit electron beams or whose unit cell heights are too small to be detected by the replica method. Therefore, a special technique is required to study the growth mechanism of such crystals. In 1958, Bassett developed a new technique of electron microscopy called the decoration method, in which minute gold grains are preferentially nucleated along steps on the surface, thus revealing the surface microtopographs of crystal faces. Step heights of one unit cell are clearly seen using the electron microscope. This method has been applied extensively to NaCl by Bassett (1958) and Bethge et al (1965, 1967, 1968). Recently, the method has been applied successfully to kaolinite and dickite by Gritsaenko and Samotoyin (1966a, b), and to synthetic hydroxyl-bearing phlogopite by Baronnet (1972). In the present study, the method has been applied to kaolin group minerals to clarify the growth mechanism of minerals formed by hydrothermal metasomatism of preexisting rocks. On nearly all the samples examined, growth spirals of mono-molecular step heights were observed, suggesting that the crystals formed by hydrothermal metasomatic replacement of preexisting solid materials are in fact grown by the spiral mechanism from a solution phase of low supersaturation. The second purpose of the present study is to correlate interlacing patterns of growth spirals with the stacking modes of unit layers. In kaolin group minerals, there are three species - kaolinite, dickite, and nacrite - whose structures differ in the stacking of kaolin layers. It is therefore expected that this difference should have been reflected as the difference in interlacing patterns of growth spirals. Interlacing patterns along the corners of growth spirals were first observed by Verma (1951) on SiC crystals, and on the basis of this observation Frank (1951) put forward a theory to account for the formation of different polytypes by the spiral mechanism. Amelinckx (1952) and Amelinckx and Dekeyser (1953) applied this idea to biotite crystals from Monte Somme, Vesuvius, and identified several higher order polytypes through the morphological analysis of interlacing patterns. Later, Sunagawa (1964) reported five-sided (hexagonal truncated one side) spirals on phlogopite crystals found in the cavities of rhyolite lava at Mutsure-Jima, Japan. He and his co-workers (1968) carried out phase-contrast microscopic studies of synthetic fluorphlogopite grown autoepitaxially from the vapor phase on cleavage surfaces of fluorphlogopite synthesized from the melt. They found that spiral morphologies were based on the stacking of unit layers with five-sided forms, through rotation of 60°, 120°, 180°, and identified 1M, 2M 1 2M2, 2O, 3T, and other higher order polytypes. Recently, Baronnet (1972) has reported in this journal several different types of interlacings on synthetic hydroxyl phlogopite. Samotoyin (1971) also observed interlaced spirals on nacrite and interpreted them in terms of translation only.Samples and Experimental Samples used in this study are as follows: Kaolinite - Itaya mine, Fukushima Pref. (thick hexagonal tabular, about lµ in diameter); Kurosawa mine, Fukushima Pref. (ill-formed hexagonal, platy to tabular, about 1 µ in diameter). Dickite - Itaya mine, Fukushima Pref. (thick hexagonal tabular from 1µ to l0µ in diameter, average from 2 to 3µ); Shokosan mine, Hiroshima Pref. (thick hexagonal tabular, a few microns in diameter). Nacrite - Yaita mine, Tochigi Pref. (well-formed thin hexagonal platy, from 5 to 30µ in diameter). The Itaya and Yaita mines are hydrothermal or hot-spring metasomatic massive-type kaolin deposits, due to Quaternary volcanism. In the Itaya mine, zonal arrangement is distinct with an inner quartz-alunite-kaolinite-dickite zone, an outer kaolinite-quartz zone and a marginal montmorillonite-cristobalite zone (Togashi and Fujii, 1972). Both kaolinite and dickite are from the inner quartz-alunite-kaolinite-dickite zones. The Shokosan mine is a much larger, hydrothermal, metasomatic, massivetype pyrophyllite-kaolinite-sericite deposit. The original rocks are dacite and its pyroclastics, and the deposits are formed by Tertiary volcanism. Zonal arrangement is distinct, and the dickite is from kaolinite ore. The Kurosawa mine is a kuroko-type1 deposit consisting of a sericite zone, a mixed-layer clay mineral zone, a kaolinite zone, and a montmorillonite zone surrounding the sulfide ore body. The kaolinite is from the kaolinite zone. Purified samples were used in this study, and were checked by X-ray diffractometry before the decoration technique was applied. The samples were dispersed in either alcohol or acetone on a cover glass, which was heated in a vacuum of 10-4 torr at 400°C for about one hour to obtain clean surfaces. Gold was then flash-evaporated in a vacuum of 10-4 torr onto the surface by heating gold grains held in a tungsten coil heater. The temperature of the sample at the time of gold evaporation was between 350 to 400°C. The amount of evaporated gold was very critical, and was judged by observing the appearance of the cover glass. Very light pink was the proper color, while light blue indicated the gold layer was too thick. After a carbon coating was applied, the sample was removed from the evaporator, and immersed in a 10 percent HF solution for about a day to completely dissolve the sample grains. After cleaning with distilled water, the thin foils were ready for observation under the electron microscope. Growth Mechanism Figure 1 is typical of the electron micrographs recorded in this study, and clearly shows that gold grains are preferentially nucleated along the steps of spiral layers. Here two questions arise: (1) whether the spiral is a growth spiral or an evaporation spiral, and (2) whether or not these layers are really monomolecular. Regarding (1), we conclude that it is really a growth spiral, since the spiral is not a depression but an elevation, judging from the inclination of the edge which is formed by the bunching of a large number of spiral layers. As to (2), it appears that the layers are probably mono-molecular, based on previous experience with NaCl (Bethge et a!, 1965, 1967, 1968) and with phlogopite (Baronnet, 1972). When layers are multi-molecular, multiple alignments of gold grains appear (Fig. 8).
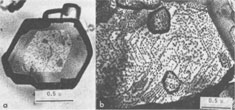
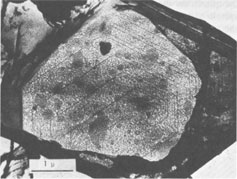
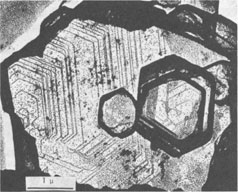
Spiral patterns are observed on all the crystals investigated, irrespective of the mineral species and the size of the crystal. Spirals have been observed to originate from an independent single-screw dislocation (Fig. 2a), from a group of dislocations (Fig. 1), from a line of dislocations (Fig. 2b), and from a pair of dislocations having opposite signs (Figs. 1, 6). Dominated spirals are also seen. In most cases, the spirals originate from real screw dislocations, not from twist boundaries or from points of contact of different crystals. Most crystals occur as independent individuals, with coalesced crystals seen only rarely. This differs from crystals grown in an open space in a moving solution since crystals grown in such environments have more opportunity to coalesce. As soon as crystals coalesce, the points of coalescence act as sites of new growth centers, from which growth layers spring out. This has been reported for CdI 2 crystals (Kitazaki, Sunagawa, and Endo, 1971), but is observed only rarely on the present specimens, suggesting that the kaolin minerals did not grow in an open space in a moving solution.As to the location of spiral centers, it is observed that the basal planes of crystals smaller than 2 microns are covered entirely by spiral layers originating from a single growth center situated near the central portion of the face, whereas larger crystals usually exhibit additional growth centers. Large nacrite crystals are typical of the latter, kaolinite of the former. Growth spirals observed on the three different kaolin minerals are in most cases elongated hexagonally (or rarely rhombic), with edges parallel to the external edges of the crystal. Near the center of the spirals, the spacings between successive arms of the spirals are wide and more or less uniform, but as the spiral layers reach the edges of the crystal, the spacings become abruptly narrower, forming six-sided vicinal faces because of the piling-up of spiral layers (Figs. 4, 5). This is seen more distinctly on crystals smaller than 0.5µ (Fig. 2a). On these minute crystals, one can usually see only one or two spiral steps, with wide marginal portions of the face occupied by densely precipitated gold grains, corresponding to the initial development of vicinal side faces. As a general tendency, spiral layers on these minute crystals are more malformed or circular than those on larger crystals. Incidentally, evidence supporting two-dimensional nucleation as the source of growth layers has not been observed even on minute crystals. All observed growth layers originate from screw dislocations.
If a crystal has a high growth rate, the spacings of successive arms of the spiral are narrow, and a large number of growth steps will be seen on the surface when growth is completed. For slow growth, the spacings will be much wider, resulting in the appearance of a small number of growth steps on the surface. Therefore, the observations described above indicate that the kaolin group minerals are formed very slowly, under low supersaturation conditions. This is consistent with the fact that most crystals exhibit polygonal spirals with wide spacings. The fact that these crystals grow by a typical spiral mechanism shows that their crystallization took place from dispersed phases of low supersaturation in which the fundamental processes were condensation, transportation, surface migration, and adsorption of the components at kink sites along the steps provided by screw dislocations. Such processes can be expected only for growth from vapor and solution phases, and not from pure melt or from solid state crystallization. In other words, the components of the kaolin group minerals were dissolved in solvents, most probably in hydrothermal solution, forming a supersaturated solution from which crystals nucleated. The supersaturation of this solution was probably very low, and the crystals grew very slowly. The crystals were not formed in an open space in a violently moving solution, but in an enclosed system under constant environment. Interlacing Patterns The three kaolin group minerals are composed of kaolinite layers, differing only in the stacking modes of the unit kaolinite layer; kaolinite has one layer, dickite two layers, and nacrite two layers (Blount, Threadgold, and Bailey, 1969). The dickite structure may be described as a regular alternation of right- and left-handed kaolinite layers (Bailey, 1963). Recent refinement of the nacrite structure by Blount et al (1969) has shown that this structure may also be described as a 2-layer form, in which alternate layers rotate 180°. Now, let us consider the morphology of growth spirals of the unit kaolinite layer, with step height of 7.2 Å. Since the surface of the unit kaolinite layer can be regarded as a hexagonal network, regular hexagonal morphology may be expected. However, the true symmetry of the kaolinite layer may result in the anisotropy of growth rates in the directions perpendicular to hexagonal edges, leading to elongated morphology rather than regular hexagonal morphology for the growth spirals. This is the fundamental morphology of growth spirals seen on the three kaolin minerals. For trioctahedral micas such as phlogopite, the spiral morphology is five sided: a hexagon truncated on one side. A theoretical analysis of spiral morphology of dioctahedral and trioctahedral micas is now being undertaken by Professor Hartman of Leiden University and by I. Sunagawa. Although the full text of this joint work will be published later, a tentative analysis suggests elongated hexagons for dioctahedral mica and five-sided forms for trioctahedral mica.
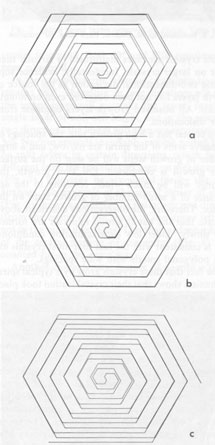
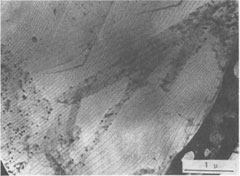
Since kaolinite consists of one-layer stacking, interlacing is not expected, and as a matter of fact, none of the kaolinite crystals exhibit interlacing patterns. Only elongated hexagonal spirals or their derivatives are seen on their basal planes (Fig. 2). Since the dickite structure consists of alternating right- and left-handed kaolinite layers, a spiral growth layer originating from a single screw dislocation with Burgers' vector corresponding to the dickite cell will split into two layers, each with the height of a single kaolinite layer. Because of different advancing rates of the upper and lower layers, one layer may overtake the other, forming a layer of unit cell height in certain directions, while in other directions the two layers advance separately. In the case of two regular triangular layers rotated 60° or 120°, as in SiC, an interlacing pattern will be formed from the beginning along six corners. Such an interlacing pattern will not be expected in this case because of hexagonal morphology. Interlacings will appear only after several turns, and until then the layer will advance separately forming tongue-like terraces. The point at which interlacing begins to appear depends on the elongation of the hexagonal form, i.e., on the anisotropy of advancing rates. Figures 3a and 3b give interlacing patterns expected for the cases in which two hexagonal spiral layers of identical elongation but rotated 60° (a) and 120° (b) originate from a single dislocation point. As can be seen in Figures 1, 4, 5, and 6, spiral layers observed on dickite crystals exhibit this type of pattern, though they differ to some extent from crystal to crystal. Both 60° and 120° rotations have been observed. In the case of nacrite, alternate kaolinite layers are rotated 180°. Therefore, in four directions out of six, the upper layer will overtake the lower, forming a single layer of unit cell height, but in the remaining two directions the layers will advance separately (Fig. 3c). Nacrite crystals invariably exhibit paired layers, or split layers in certain directions, which is quite different from the spiral layers seen on dickite crystals (Fig. 7). This may be accounted for by the above analysis. An example of the spiral center of nacrite (Fig. 8) shows an interlacing pattern in one direction (from the center of the spiral to the upper left), but not in the other directions. The observed morphology of the central portion of the spiral (Fig. 8) is accounted for in the same way. Another characteristic feature of nacrite is the occurrence of triangular or rhombic terraces (Fig. 9) at the marginal portions of spirals near the crystal edges. They are not seen on kaolinite and dickite. This sort of terrace is universally observed on the surface of well developed crystals, examples having been reported on hematite (Sunagawa, 1960) and SiC (Sunagawa, 1974), and is formed by the bunching of thin layers because of an impurity adsorption or the formation of stacking faults. Near imperfections, the advancing layers bunch together to form thicker layers and a terrace-like pattern. Judging from the straight crystallographic lines at the upper side, the defects causing the bunching are most probably stacking faults.
Such terraces are not seen on kaolinite and dickite because of the small crystallite sizes. Since three kaolin minerals are polar with an oxygen layer on the bottom and a hydroxyl layer on top, we tried to find differences of surface microtopographs between (001) and (001) faces, as in sphalerite crystals (Komatsu and Sunagawa, 1965). However, we could not establish such differences with certainty, because it was impossible to turn over the samples for this investigation. Acknowledgments The writers are indebted to Professor T. Takeuchi, Dr. K. Yamaoka of Tohoku University, and Mr. Y. Togashi of the Geological Survey of Japan for supplying the specimens. Thanks are also due to Professor Robert E. Newnham, Pennsylvania State University, for correcting the English of the original manuscript. References AMELINCKX, S. (1952) La croissance helicoidale de cristaux du biotite. C. R. Acad. Sci. Paris, 234, 971-973. ______, and W. Dekeyser (1953) Le polytypisme des mineraux micaces et argileux. C. R. XIXth Congr. Geol. Int., p. 9-22. BAILEY, S. W. (1963) Polymorphism of the kaolin minerals. Am. Mineral. 48, 1196-1209. BARONNET, A. (1972) Growth mechanisms and polytypism in synthetic hydroxyl-bearing phlogopite. Am. Mineral. 57, 1271-1293. BASSETT, G. (1958) A new technique for decoration of cleavage and slip steps on ionic crystal surfaces. Phil. Mag. 3, 1025-1042. BETHGE, H. (1967) Electron-microscopic investigations of the molecular processes during evaporation and growth of crystals. Crystal Growth (Proc. ICCG-1, Boston), p. 623-627. ________, AND M. KROHN (1965) Wachstumsvorgänge auf NaCl Kristallen nach Anlösung. Adsorption et croissance cristalline. C. N. R. S. 152, 389-406. ________, K. W. KELLER, AND E. ZIEGLER (1968) Molecular processes during evaporation and growth of crystals. J. Crystal Growth, 3-4, (Proc. ICCG-2, Birmingham), p. 184-187. BLOUNT, A. M., I. M. THREADGOLD, AND S. W. BAILEY (1969) Refinement of the crystal structure of nacrite. Clays Clay Minerals, 17, 185-194. FRANK, F. C. (1949) The influence of dislocations on crystal growth. Disc. Farad. Soc. No. 5, 48. _________ (1951) The growth of carborundum: Dislocations and polytypism. Phil. Mag. 42, 1014-1021. GRITSAENKO, G. S., AND N. D. SAMOTOYIN (1966a) The decoration method applied to the study of clay minerals. Proc. Int. Clay Conf., Jerusalem, Israel, 3, 391-400. ___________, AND ________ (1966b) Decoration method applied to the study of the relationship between microcrystal surface microtopography and the crystal structure of kaolinite and dickite. VI. Int. Congr. Electron Micros. Kyoto, Japan. KOMATSU, H., AND I. SUNAGAWA (1965) Surface structures of sphalerite crystals. Am. Mineral. 50, 1046-1057. KITAZAKI, S., I. SUNAGAWA, AND Y. ENDO (1971) Growth and dissolution of cadmium iodide crystals. Mineral. Soc. Japan, Spec. Pap. 1 [Proc. IMA . IA GOD Meet. '70], p. 109-113. SAMOTOYIN, N. D. (1971) Study of nacrite by vacuum decoration method. Ist'. Acad. Nauk. CCCP Ser. Geol. 10, 114-126 (in Russian). SUNAGAWA, 1 (1960) Mechanism of crystal growth, etching, and twin formation of hematite. Mineral. J. 3, 59-89. _________ (1961) Step height of spirals on natural hematite crystals. Am. Mineral. 46, 1216-1226. _________ (1962) Mechanism of growth of hematite. Am. Mineral. 47, 1139-1155. _________ (1964) Growth spirals on phlogopite crystals. Am. Mineral. 49, 1427-1434. _________ (1974) Surface micro-topography of silicon carbide. Sci. Rep. Tohoku Univ. 12, 239-278. Y. ENDO, N. DAIMON, AND I. TATE (1968) Nucleation, growth, and polytypism of fluor-phlogopite from the vapour phase. Crystal Growth 1968 (Proc. ICCG-2, Birmingham, U.K.), p. 751. TOGASHI, Y., AND N. FUJII (1972) Study on the Itaya kaolin deposit, Yamagata Prefecture, Northeastern Japan. Bull. Geol. Surv. Japan, 23, 595-612 (in Japanese). VERMA, A. R. (1951) Observations on carborundum of growth spirals originating from screw dislocations. Phil. Mag. 42, 1005-1013. ________ (1956) A phase contrast microscopic study of the surface structure of blende crystals. Mineral. Mag. 31, 136. Manuscript received, September 11, 1974; accepted for publication, December 13, 1974.NOTE 1 "Kuroko-type" ore deposits are massive or sometimes stratified chalcopyrite-sphalerite-galena-barite ore deposits of syngenetic origin, typically occurring in certain horizons of Tertiary age in Northeastern Japan. [var:'startyear'='1975'] [Include:'footer.htm'] |