 | The Mineral Identification Key |  |
 | The Mineral Identification Key |  |
Cleavage refers to the way some minerals break along certain lines of weakness in their structure. Mica is a good example breaking along very closely spaced flat planes that yield thin "sheets." Calcite is another good example, breaking along three different planes that yield blocky fragments that look like a rectangular box that has been warped called a "rhombohedron" or, simply, "rhomb." Galena breaks along three planes at right angles to one another, producing true cubes as fragments.
Cleavages are described in terms of their quality - how smoothly the mineral breaks - and their difficulty - how easy, or how hard, it is to produce the cleavage. The quality of cleavages are perfect, imperfect, distinct, good, fair, and poor. The difficulty is described as easy, hard, and difficult to produce. By way of examples, the micas have a perfect cleavage in one direction that is easy to produce; calcite has a perfect cleavage in three directions that is also easy to produce; the feldspars have a perfect cleavage in one direction that is easy to produce and a good cleavage in another direction that is hard to produce; and diamond has a perfect cleavage in four directions that is easy to produce. Sphalerite has perfect cleavages in six directions, some of which are easy to produce, others hard - hence you won't always see all six cleavage surfaces in any given sample of the material.
Microcline feldspar,
South Dakota, 4cm long.
Perfect cleavage is the right face.
Good cleavage is the top face
Cleavage may also be described in terms of crystallographic type:
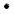
cubic (galena)
Galena, 2 cm across, Australia
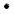
octahedral (fluorite)
Fluorite, 5cm across, S. Illinois
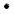
rhombohedral (calcite)
Rhodochrosite, 10 cm across, Colorado
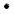
prismatic (amphiboles)
Spodumene, 3cm tall, California
pinacoidal or basal (micas)
Muscovite, 2 cm across, South Dakota
etc.These are usually referenced to what are called crystallographic forms, usually using a shorthand known as Miller Indices. This Key does not get that advanced, but the guides many collectors use often have this information in them.
The main thing that needs to be considered in the identification of minerals is whether or not a sample has a cleavage many minerals dont, breaking without producing smooth flat surfaces. Next is whether or not there are two or more cleavage surfaces present at angles to one another and, if so, the quality of the various cleavages. Where two or more cleavage surfaces are present, it then becomes important to figure out which crystal form they represent cubic, prismatic, and so on. This is usually done by "guestimating" the angles between cleavage surfaces. Some are easy to see, like galena with its three perfect cleavages at 90 degrees to one another being cubic. Others can be hard to determine and may require measurement of the angles. A device called a contact goniometer can be handy for doing this. It is simply a protractor with an adjustable arm on it that is used to lay along one cleavage surface while the base of the protractor is laid across another. More information on this can be found in some field guides and most mineralogy texts. One can also make simple line drawings on a sheet of paper of the various angles common to minerals and keep the sheet in your guide. This can be used for making "eyeball comparisons" with the angles between cleavage surfaces on samples.
By-and-large, cleavages at 90 degrees to one another indicate a cubic form, cleavages at 120 and 60 degrees in the same sample indicate a rhombohedral form, and cleavages at acute to obtuse angles over long surfaces indicate a prismatic form such as in amphiboles. Nearly rectangular or sharp angles in prismatic minerals may indicate a Pyroxene Group mineral, while more open angles approximately 120 degrees may indicate an Amphibole Group mineral. (Not all do, but these three groups are common and frequently seen, so seeing these types of cleavages is likely to mean you have one of them.) A single cleavage at 90 degrees to a crystal face indicates a basal form such as in micas. See a good guide book for further information.
Parting: Some minerals which do not exhibit cleavage do have a characteristic that is similar, called parting. It occurs in minerals that are crystallographically twinned, or which have been stressed by pressure. It is usually not as well, or regularly, developed as cleavage surfaces - resembling an indistinct or poorer cleavage, and it is hard to difficult to produce in specimens. Perhaps the three best examples of it are the basal parting seen in the pyroxene minerals, the micaceous appearing parting in "specular" hematite, and the rhombic parting seen in corundum. But the fact is that for all practical purposes it looks just like cleavage - you only know it isn't when you read in books that what you are seeing is due to twinning or pressure.
Return to Step 6 Return to Step 8 Return to Step 11 Return to Step 13 Return to Step 14
[ Table of Contents ] [ Introduction ] [ Identification Kit ] [ Mineral Properties ] [ Environments & Associations ] [ In Conclusion ] [ The Mineral ID Key ]